Report

Executive summary
This report outlines the strategic path for India to become a global pharma powerhouse in the near term (2030) and beyond (2047, coinciding with the 100th year of India’s independence). Developed in consultation with industry leaders and government stakeholders, the report assesses the Indian pharmaceutical industry’s current global position, identifies future opportunities, and highlights key imperatives for growth.
Written in collaboration with
Written in collaboration with

With the Indian economy growing rapidly, the government has set an ambitious target for the nation to become a $30 trillion to $35 trillion economy by 2047. The pharmaceutical industry, with its robust global footprint, is a critical component of this growth strategy. It is currently the fifth-largest contributor to India’s manufacturing GVA, generating a healthy trade surplus and supporting numerous livelihoods. India, already the largest global supplier of generic medicines, fulfills approximately 20% of global generics demand. Yet it still lags from a value perspective in the global pharmaceutical market. This underscores the need to transition from a volume-based exporter to a value-driven leader in high-value products, such as biosimilars and innovative therapies.
Several key trends are shaping the “Indian pharmaceuticals for the world” story: namely, the drive for supply chain resilience, enhanced focus on R&D and quality, shift toward value through contract development and manufacturing organizations and contract research organizations (CDMOs and CROs), increased regulatory harmonization, robust funding for Indian pharmaceuticals, the rise of digital and AI tools, and the push for sustainability. Riding these trends, India’s pharmaceutical exports are projected to grow 10- to 15-fold, reaching nearly $350 billion by 2047.
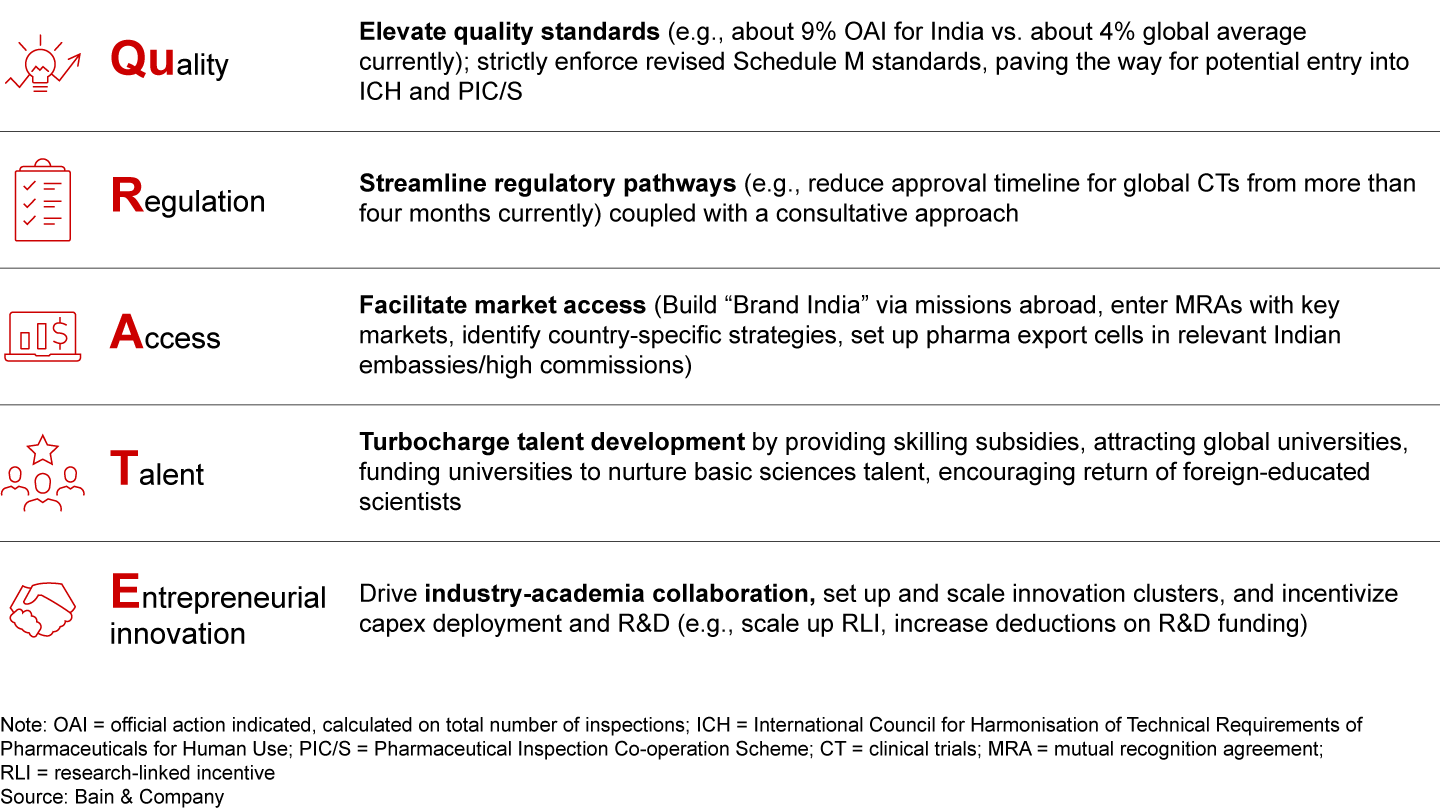
To achieve this, India must chart a granular view of the global demand landscape, targeting pockets of opportunity for exports within specific geographical clusters or countries. By focusing on the mantra “QuRATE,” which encompasses five overarching pillars—Quality, Regulation, Access to global markets, Talent, and Entrepreneurial innovation—India can stand out and capture these opportunities. India must also focus on several key drivers across segments: expanding exports of specialty generics, boosting API exports and CDMO services while leveraging the China+1 theme, expanding vaccine exports to high-income countries, and capitalizing on the biosimilars and innovative products opportunity, to name a few.
Collaboration between the private sector and the government is essential to realizing this vision. The time to act is now, as transitioning from volume to value will be critical for India to secure its place among the world’s top pharmaceutical exporters.
Introduction: India’s time is now
India stands on the threshold of significant transformation. The nation’s GDP has jumped from $1.9 trillion to $3.6 trillion over the past decade, with the Indian economy overtaking the UK’s in 2024 to occupy fifth place in the world GDP order. The Indian government aims to become a $30 trillion to $35 trillion economy by 2047, positioning India as the third-largest economy, surpassing Japan and Germany.
Among the many sectors crucial to realizing this ambitious goal, the Indian pharmaceutical industry holds a pivotal position. As the fifth-largest contributor to manufacturing GVA, it drives approximately 4% of India’s foreign direct investment (FDI) inflows, sustains a $19 billion trade surplus, and supports 2.7 million livelihoods, either directly or indirectly.
The global pharmaceutical market currently stands at about $1.6 trillion, with the Indian market valued at approximately $55 billion. This is expected to grow 2.2 to 2.4 times by 2030, reaching $120 billion to $130 billion in value and increasing India’s share from 3% to 3.5% currently to nearly 5% by 2030. The Indian pharma market is unique in that its export market is as large as its domestic market, and both are expected to grow in the foreseeable future. Indian pharma exports currently play a pivotal role in the growth of the economy, accounting for 6% of India’s total merchandise exports by value.
India has a strong pharma network, with more than 10,000 manufacturing facilities, over 3,000 pharma companies, and 650 US-FDA-compliant pharma plants—the largest number outside the US. As the largest supplier of generic medicines globally, supplying one in five generic drugs sold, India has earned the title “pharmacy of the world.” It currently ranks third in the world in terms of exports by volume (see Figure 1), up from seventh in 2019. India’s pharma supplies reach approximately 200 countries and territories worldwide, supplying close to 50% of Africa’s requirement for generics and 40% of US generics demand. That success is underpinned by India’s ability to produce high-quality, low-cost medicines.
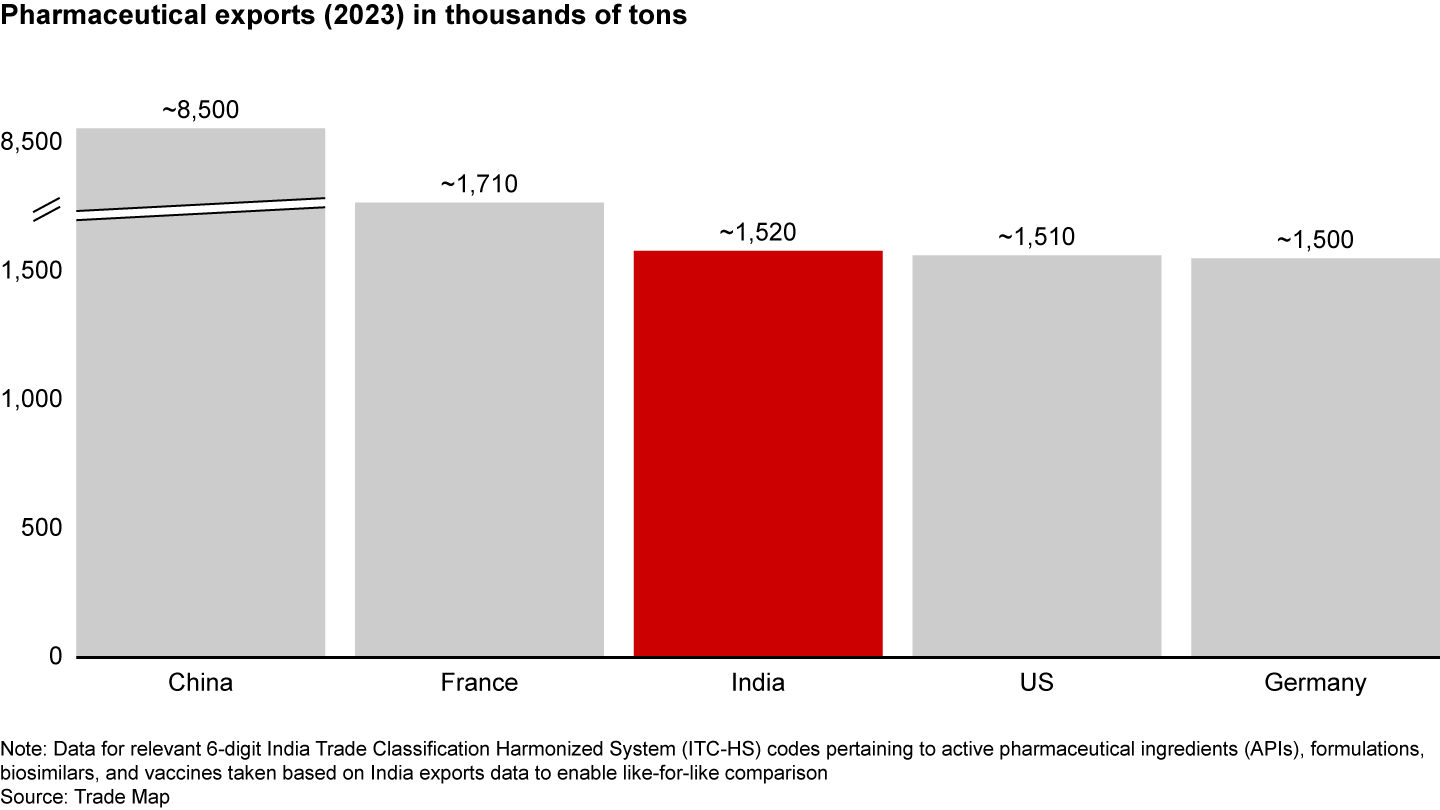

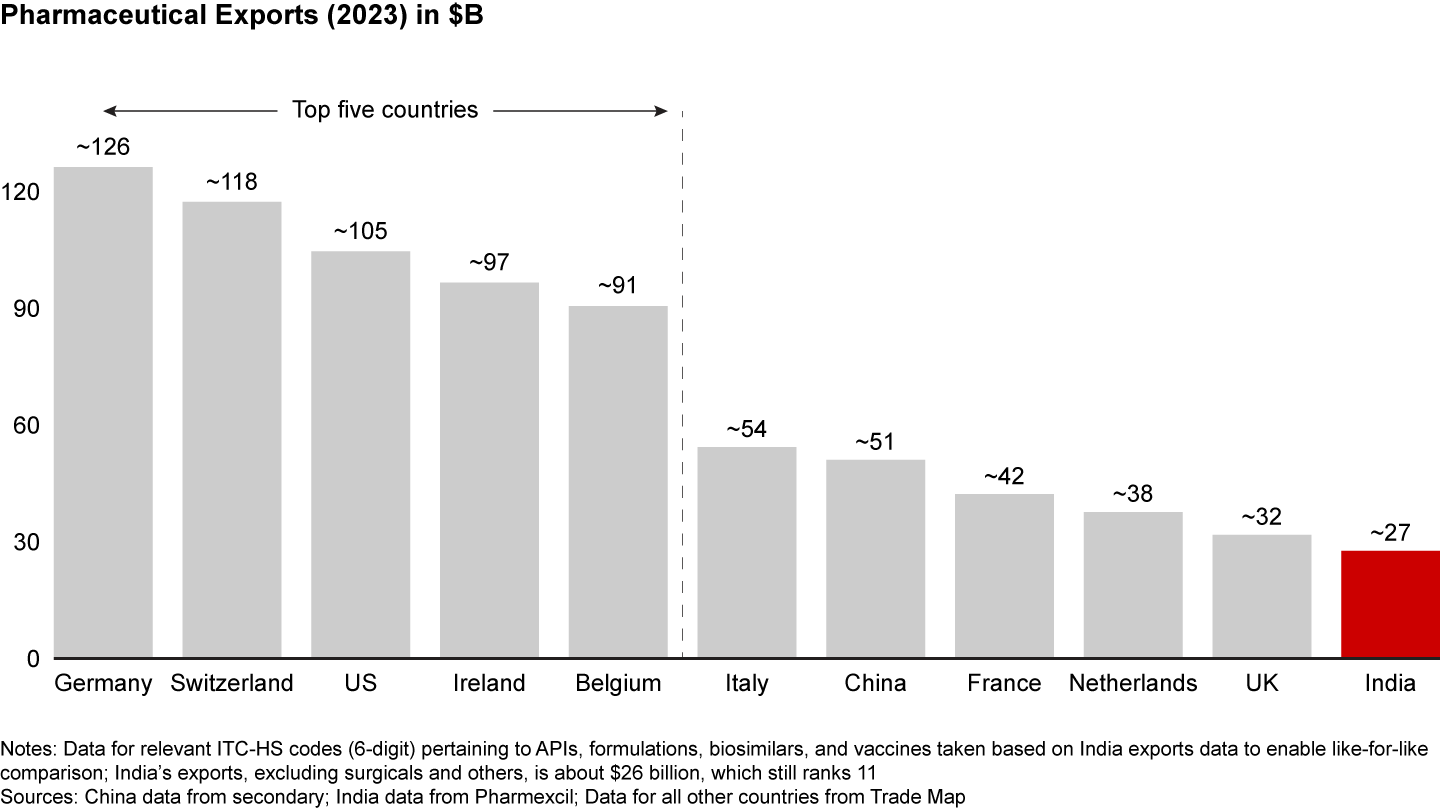
However, given India’s historical focus on commodity generics, it still has work to do before it can claim to be a leader in the value of exports. Indian pharmaceutical exports rank 11 on the list globally in value terms (see Figure 2), accounting for 3% of total pharmaceutical exports. Undoubtedly, there is room for growth here; by innovating and upgrading the export basket to include specialty generics, biosimilars, and innovative products, the industry can drive the transition from volume to value, potentially achieving its ambition of being ranked in the top five nations in terms of value of exports by 2047. Given long product life cycles and long development lead times, the industry and the government must act now to seize this opportunity.

Megatrends shaping Indian pharma for the world
Seven major market trends play into India’s quest to become a global pharmaceutical export hub (see Figure 3). These trends have the potential to strengthen India’s standing in the global market and enhance its reputation as a reliable and innovative supplier.


1. Supply chain resilience: Covid-19 laid bare the shortcomings of globalization by disrupting the flow of pharmaceutical supplies to key geographies. This has driven developed economies like the US and the EU to localize pharmaceutical production to enhance supply chain control. While reshoring gains traction, companies are also exploring near-shoring in eastern and southeastern Europe, seeking the twin benefits of cost advantage and a higher degree of control than is possible with offshore production. Additionally, international strategic efforts, such as Quad (involving the US, India, Australia, and Japan) and the Indo-Pacific Economic Framework (IPEF) Supply Chain Agreement, aim to build secure and diversified global supply chains through increased collaboration.
There is also a growing push to reduce reliance on single sources. Recent legislative efforts, such as the US Biosecure Act, restrict partnerships with major Chinese biotech firms. India stands to benefit, offering superior service levels, cost advantage, and a larger talent pool.
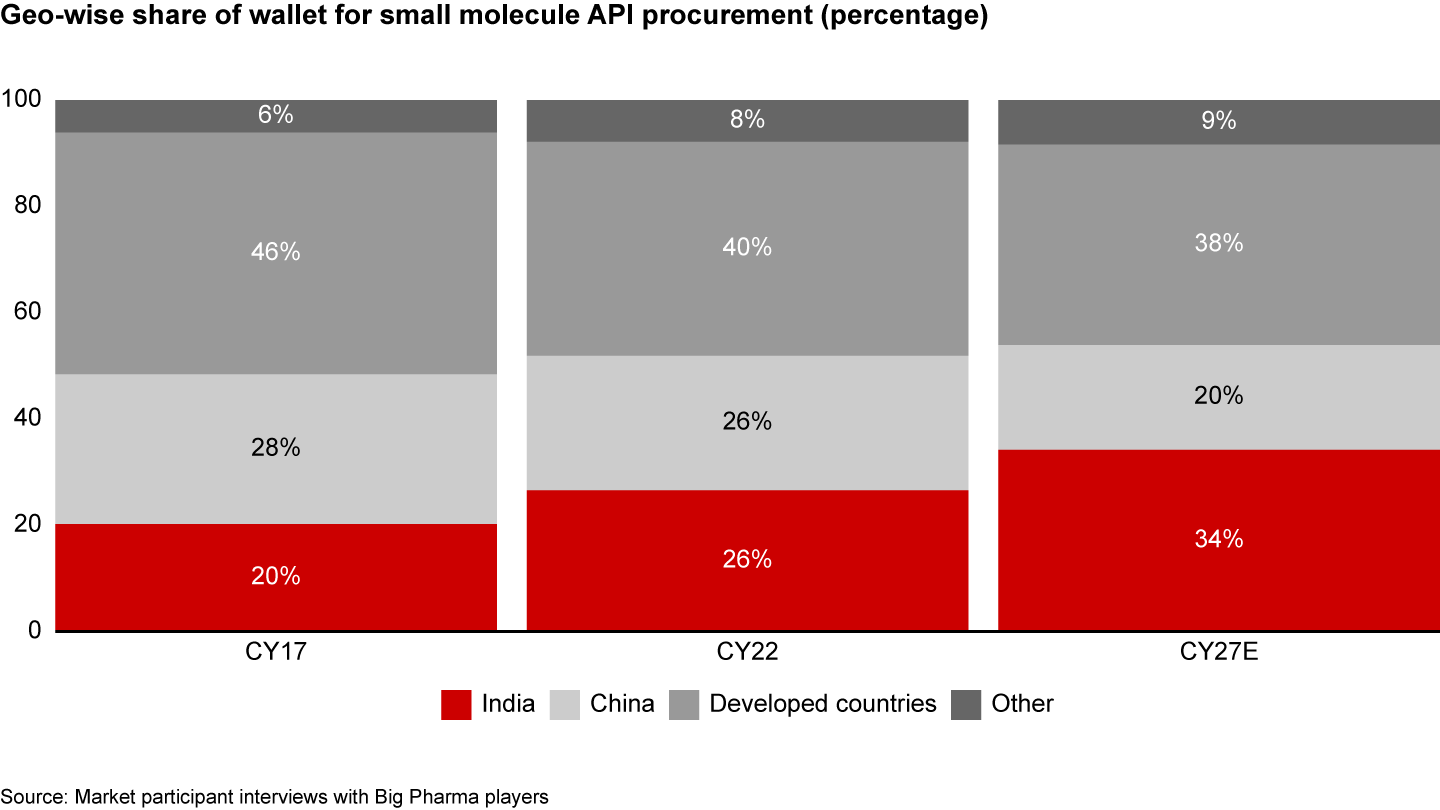
It’s also helpful that top Indian API manufacturers have performed better on quality measures over the past decade. Among all the inspections conducted by US-FDA, the average No Action Indicated (NAI) was seven percentage points higher for Indian companies than for the top Chinese producers. Interviews with Big Pharma players also convey this theme with a proposed strategic shift of share of wallet to India (see Figure 4).

To capitalize on this, India is intensifying its “Make in India” focus through production-linked incentives (PLIs). Close to $3 billion has been earmarked for pharmaceutical and medical devices, which has attracted investments worth nearly $4 billion as of April 2024. There is also an opportunity to strengthen these programs to address India’s continued dependence on a single source for bulk drug imports. Approximately 72% of overall bulk drug and intermediates imports by India in FY24 are from China, vs. 66% in FY22. Industry participants have also called for greater allocation of these incentives to produce biologics, biosimilars, and cell and gene therapies instead of simply using them to manufacture generic drugs. Further tweaks in eligibility criteria will be required to allow CDMO/CROs to receive PLI benefits. The primary determinants for eligibility—the number of ANDAs and DMFs—do not apply to CDMO/CROs. Continued support for and expansion of PLI will be critical for India to benefit from its current geopolitical and demographic advantages.
2. No compromise on R&D and quality: Top Indian pharma companies have consistently increased their R&D spending year over year, focusing on high-value products. India’s top 10 players have a robust pipeline comprising over 40 NCEs/NBEs, signaling a strong push toward innovation.
At the same time, global quality standards are becoming stricter, with regulatory bodies imposing harsher penalties for non-compliance. One example is the US-FDA’s move to impose civil penalties for incomplete clinical trial data. India has made significant strides in meeting international quality standards, with its share of US-FDA inspections resulting in Official Action Indicated (OAI) declining from 19% in 2013 to 9% in 2023 (though still above the global average of 4%). Revisions to Schedule M in 2023 are expected to further improve compliance, boosting India’s adoption of specialty generics and innovative products.
“India’s manufacturing costs are the lowest in the world; however, compliance remains a key factor to grow pharma exports. We have embraced digital interventions across our plants to eliminate errors and ensure consistent quality.”
3. Shift toward value through CDMOs/CROs: Pharmaceutical companies have historically outsourced manufacturing to Indian CDMOs due to capacity constraints or end-of-life cycle stages. However, with improved drug development and R&D capabilities provided by CDMOs and CROs, outsourcing now covers more of the value chain, from research to clinical trials. Indian CDMOs and CROs are increasingly focusing on high-value, innovative products. Further, Indian companies are out-licensing their own IP-driven products to front-end partners, thus capturing a larger chunk of the value chain. The growth of CDMOs and CROs can create a multiplier effect in innovation talent, with the next generation of biotech companies likely to emerge from those exposed to innovative thinking.
“Our company is actively shifting focus to innovation and on-patented products, which is reflected in approximately 50% of our FY24 revenue coming from innovation-related work.”
4. Growing regulatory harmonization: Regulatory harmonization—aimed at simplifying and standardizing regulations to accelerate the pace of approvals—is happening globally. For instance, the African Medicines Regulatory Harmonization Program facilitates harmonized technical and regulatory guidelines for member states. Additionally, the US-FDA’s Project Orbis initiative provides a framework for concurrent submission and review of oncology products among international partners, which promises quicker approval of oncology drugs. The European Medicines Agency (EMA) also promotes regulatory harmonization across the EU through policies like centralized procedure (mandatory for most innovative products), which enables a single EU-wide assessment and grants marketing authorization valid in all member states.
Regulatory harmonization across nations can ease India’s entry into key markets by simplifying compliance, streamlining approvals, and reducing duplication of work.
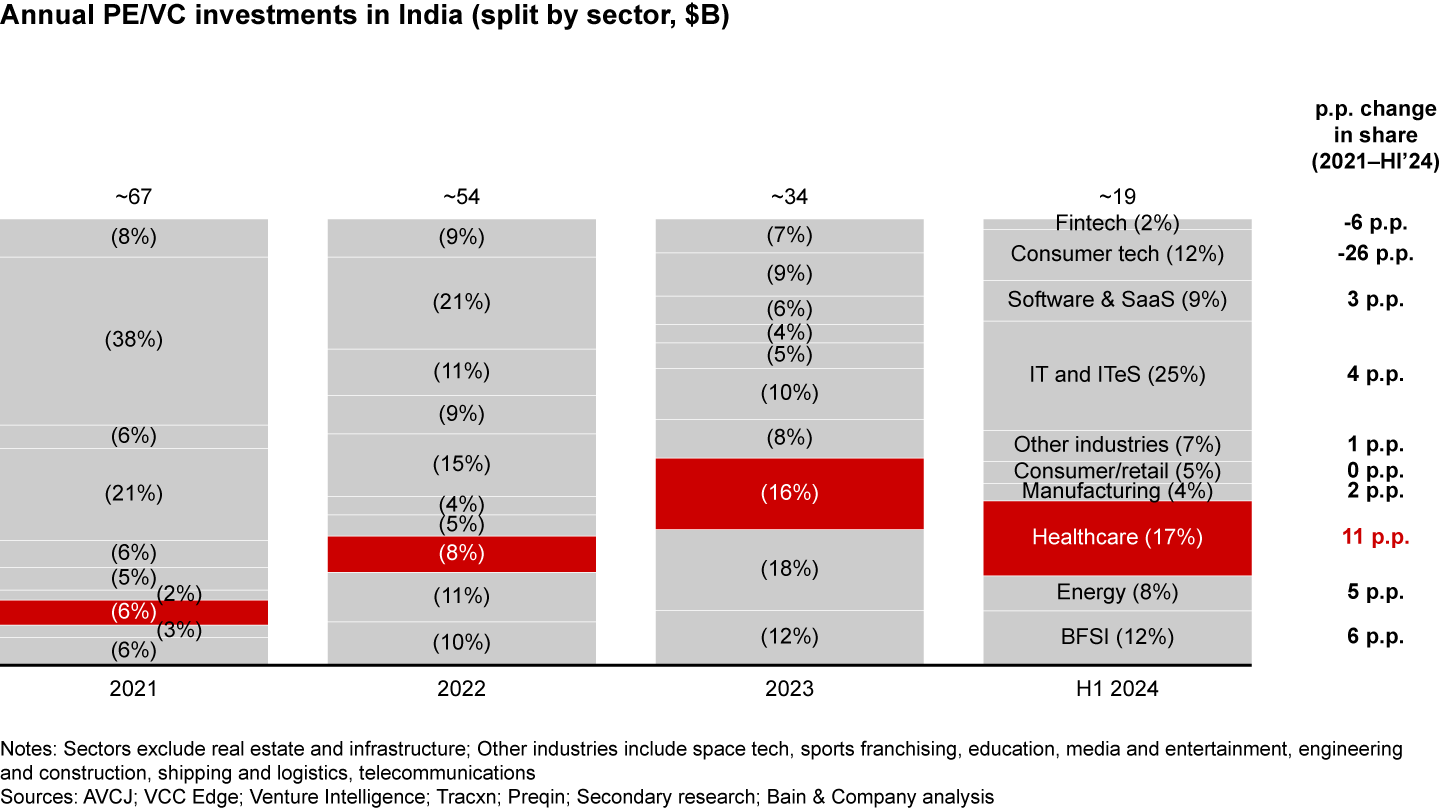
5. Robust funding for Indian pharmaceuticals: Healthcare’s share of Indian PE/VC investments has grown from 6% in 2021 to 17% in the first half of 2024, which is the highest increase across sectors (see Figure 5). PEs are setting up robust platform plays across the value chain by acquiring companies and realizing synergies among them. For instance, Advent created Cohance with API and high-value specialty chemistry players, later adding CDMO capabilities through acquisitions. Similar platforms have been set up by Carlyle and PAG. Such investments are likely to pave the way for Indian players to compete on the global stage.
Besides PE activity, there is a growing VC interest in the biotech sector. India is one of the top 12 destinations for biotechnology worldwide, with a 2024 industry valuation of $130 billion that is expected to rise to $300 billion by 2030. India’s nascent biotech ecosystem includes more than 800 core biotech companies, over 75 bio-incubators, and over 6,000 biotech start-ups. The number of biotech start-ups can potentially reach more than 10,000 by 2025 as funding continues to increase. This investment, along with the focus on R&D, will encourage the growth of Indian biopharma capabilities.

6. Rise of digitalization and generative AI: IT and pharmaceuticals are two sectors where India has a significant competitive advantage. When the two are merged, India has an opportunity to become a powerhouse. Digitalization is changing every facet of the pharma value chain—from Industry 4.0-driven plants that reduce the potential for human error in manufacturing and quality while improving machine uptime and efficiency, to cloud-based supply chain solutions that enable digital planning and procurement.
Generative AI is also rapidly advancing in healthcare, with applications in R&D, manufacturing, sales and marketing, and support functions. In drug discovery, it accelerates literature review, hit finding, and lead optimization. Clinical use cases include trial doc generation, regulatory authoring, and adverse event processing. Indian companies are exploring AI-driven drug discovery, potentially boosting India’s role in innovative products. A Bain survey of 85 industry leaders shows combining generative AI with traditional AI could add two times more value over three to five years vs. just using traditional AI. Though India’s generative AI presence in pharma is nascent, its adoption could accelerate innovation and improve export competitiveness.
7. Push for sustainability: Globally, sustainability is becoming a major focus across industries, with ESG tracking becoming more defined and entities setting ambitious targets for reducing waste and carbon emissions. For instance, the EU advocated for including new Trade and Sustainable Development chapters in all trade agreements, which may include possible trade sanctions for breaches of the Paris Climate Agreement and labor principles set by the International Labor Organization. The pharmaceutical industry also adopted ESG practices. Canada has proposed FDA amendments that prohibit drug sales without adequate environmental risk assessment; the UK’s NHS aims to reach net-zero emissions by 2040; and global pharma giants, such as Novartis and GSK, are targeting net zero across their value chains by 2040 to 2045.
These global measures are pushing Indian pharmaceutical companies to strengthen ESG strategies. Indian firms like Sun Pharma, Dr. Reddy’s, and Cipla have made positive strides here. However, broader adoption of ESG practices like waste management and product end-of-life cycle management are essential for long-term global competitiveness.
Indian pharma exports: An approximately $350 billion opportunity by 2047
The Indian pharma market, currently valued at $55 billion, is expected to reach $120 billion to $130 billion by 2030 and to approach $450 billion by 2047.
In 2023, Indian exports amounted to $27 billion—a jump from $19 billion in 2018—reflecting a growth of 8% p.a. Most of these exports (more than 70%) are formulations, with bulk drugs and drug intermediates comprising approximately 20% (see Figure 6). Other export segments include vaccines, biosimilars, and innovative products (NCEs, NBEs).
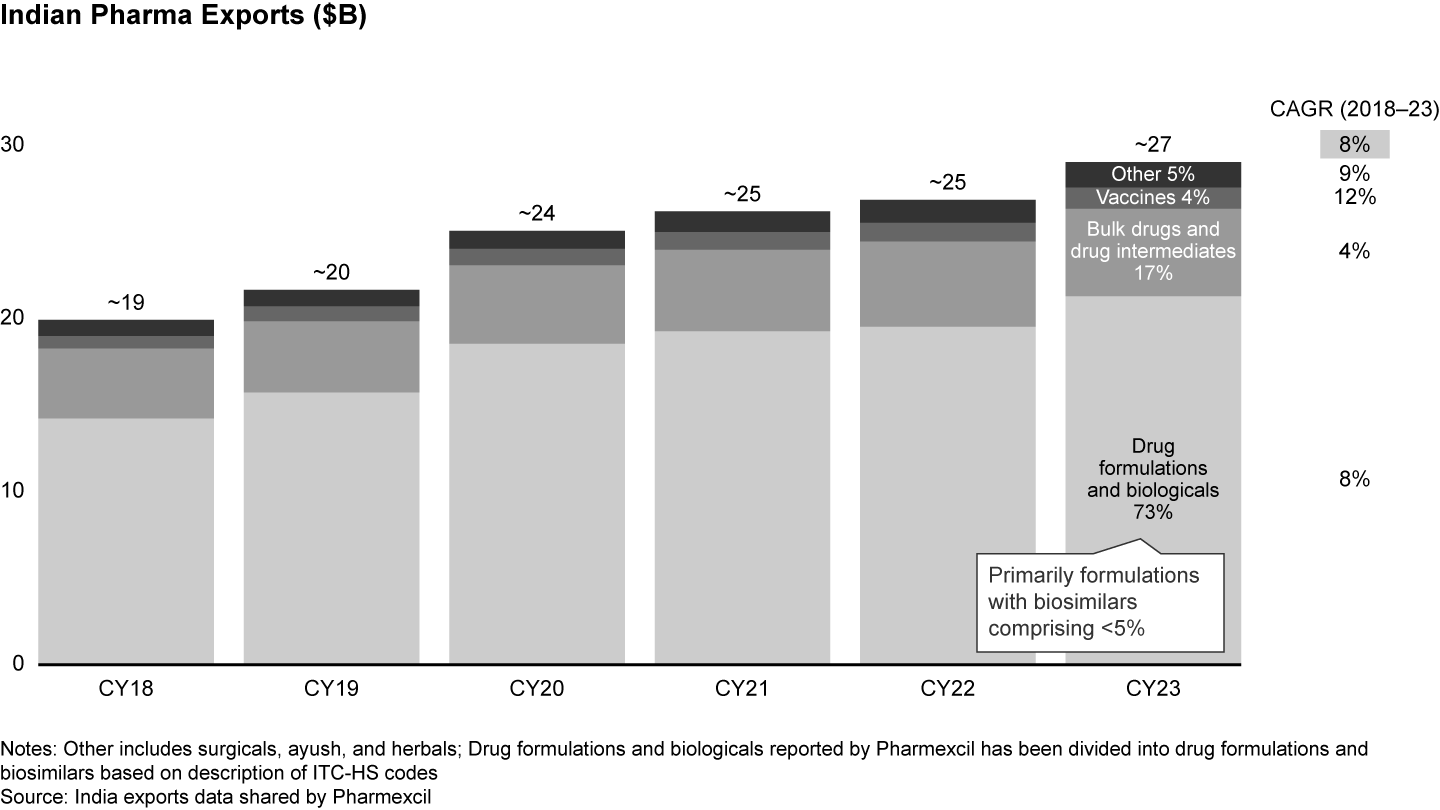

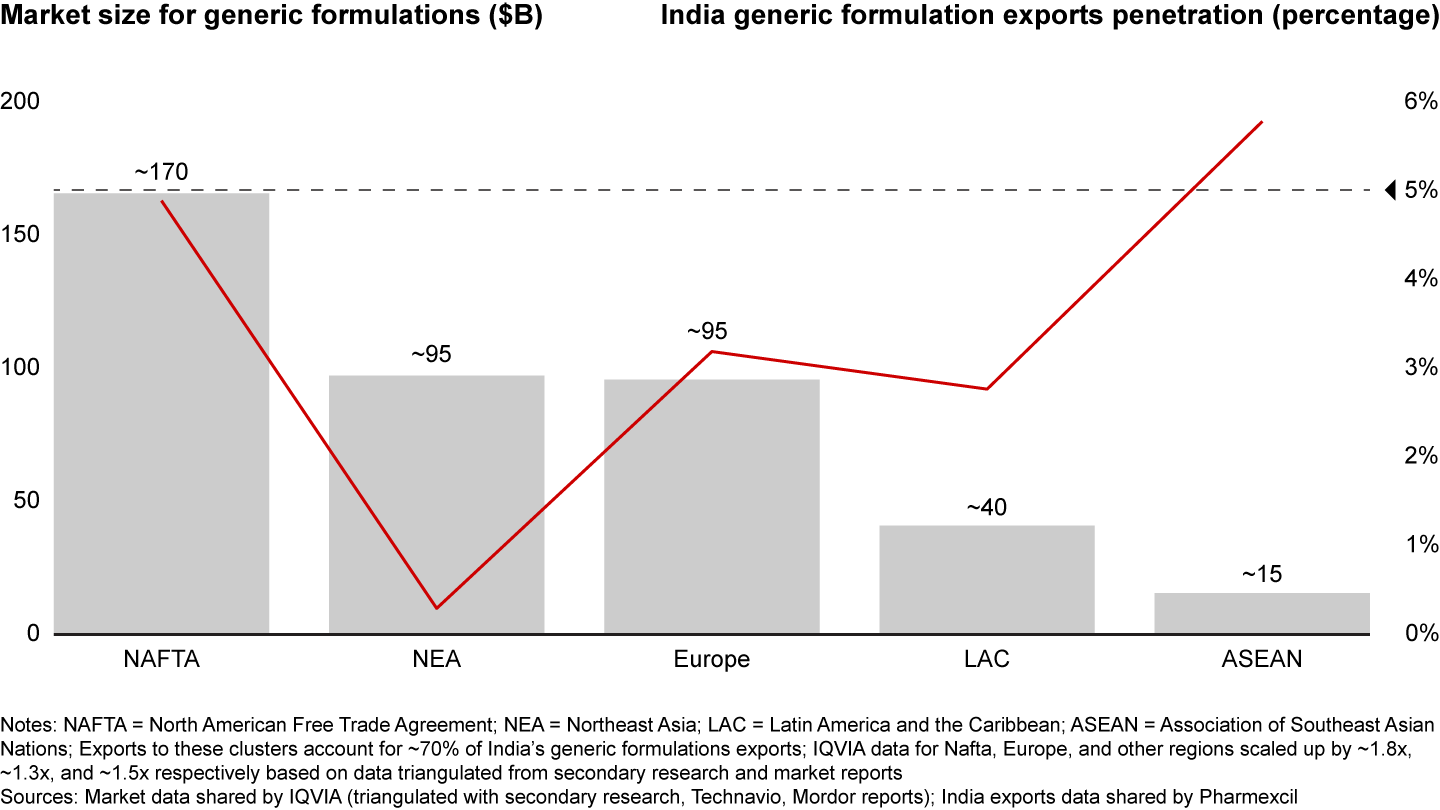
However, India’s pharma exports are underrepresented in most major markets. The market penetration of generic formulations exports, in terms of value, is below 5% in NAFTA, Europe, NEA, and LAC which together comprise more than 80% of the market (see Figure 7). While India is already focused on NAFTA, doubling down on Europe and NEA can drive growth. Since Europe is only 70% genericized, compared to the US at 90%, a sizeable opportunity exists in the EU. There is also room for growth in Indian biosimilars exports to NAFTA and Europe, where current exports by value comprise just 1.5% to 2% for NAFTA and 1% to 1.5% for Europe, signaling headroom for growth.

There is also a great deal of potential to boost exports across segments. For instance, value growth in formulations is expected to stem from higher penetration of specialty generics in target markets. The China+1 theme is likely to boost Indian API exports and CDMO services. Vaccine exports are also expected to reach new heights by focusing on middle-income countries (MICs) in the short term and expanding into high-income countries (HICs) over the long term. The shift from focusing purely on pediatric vaccines to including adult vaccines will also drive growth. Investments in biosimilars are also expected to start yielding dividends in the coming decade. We predict the export of biosimilars to follow a similar trajectory as small molecule generics with a lag of nearly a decade. Exports of NCEs and NBEs are still nascent, but a focus on building R&D capabilities and targeting high-growth new age treatment paradigms, such as cell and gene therapies, will be pivotal to the sustained long-term growth of these innovative products.
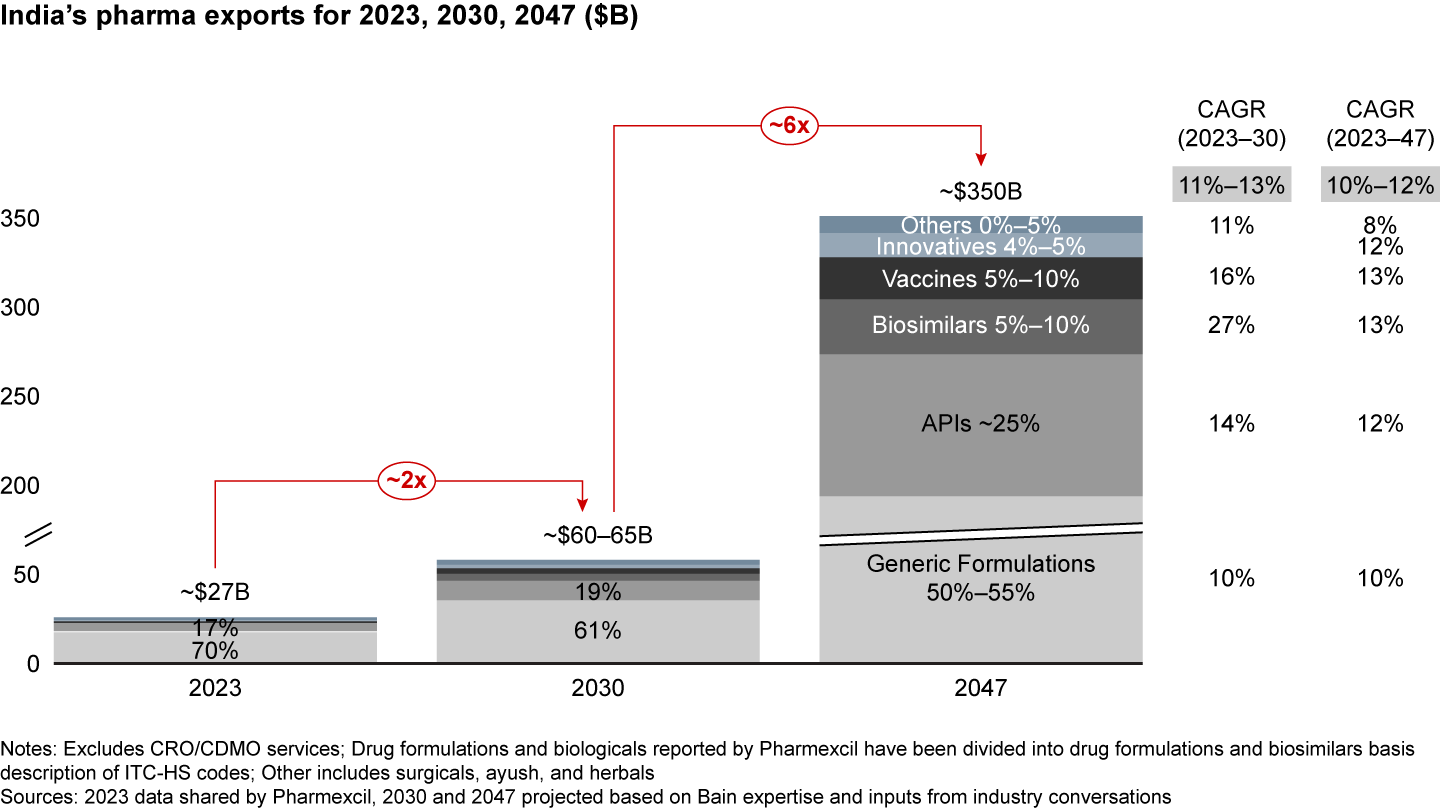
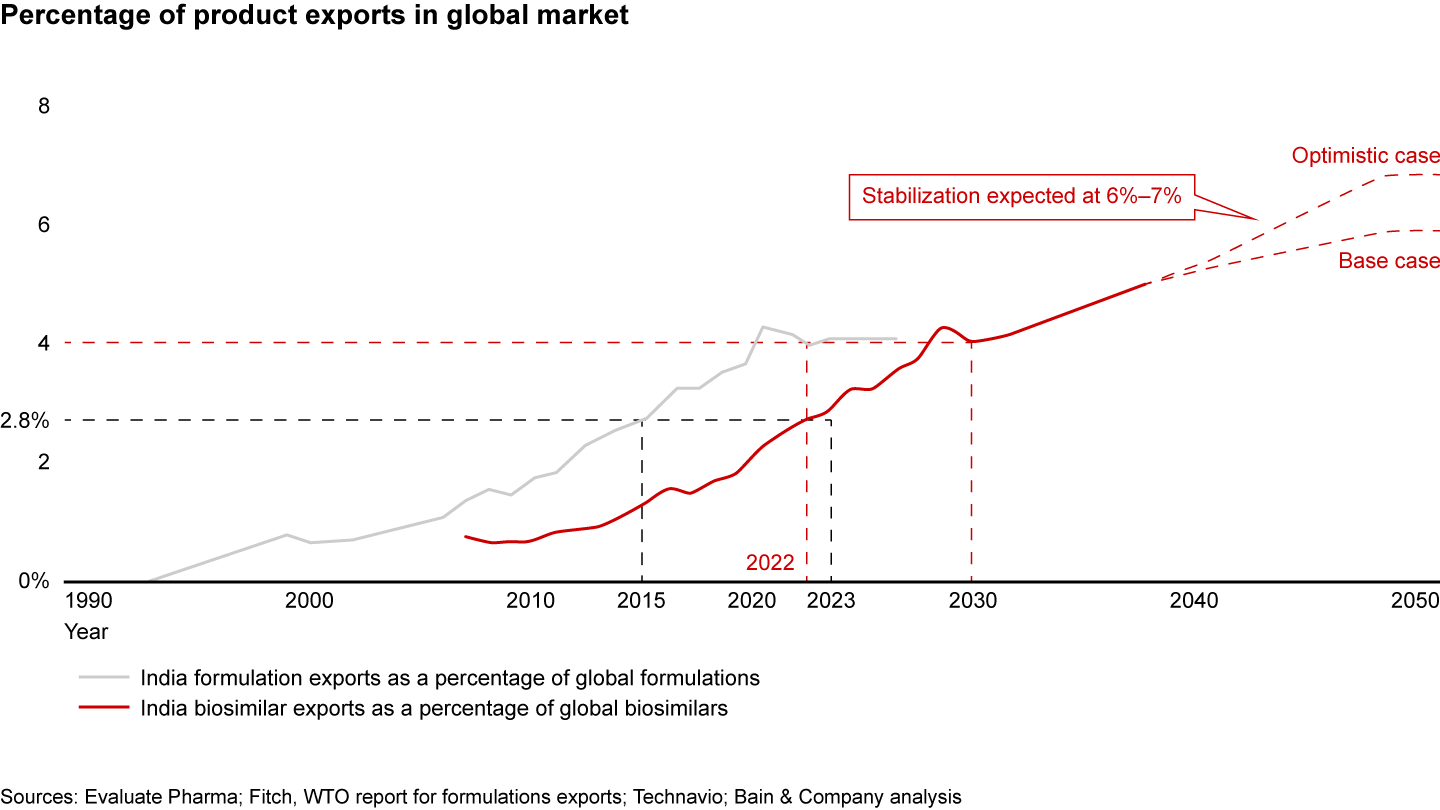
India has a unique opportunity to more than double its pharmaceutical exports to $60 billion to $65 billion by 2030. If it focuses on the right drivers, it could even reach $350 billion by 2047, increasing its output sixfold (see Figure 8). This suggests that pharma’s share in India’s merchandise exports will increase to about 7% by 2047 from about 6% currently. Moreover, the share of high-value, complex products such as biosimilars and innovative products in India’s export basket will need to increase from less than 3% currently to 10% to 15% by 2047.

API and generics: Strengthen the core
The API and generic formulations market is poised for significant growth. Global API consumption is valued at close to $240 billion and is expected to grow by 6% p.a. to reach $350 billion by 2030. This will be driven by several factors, including the growing geriatric population; a rising prevalence of lifestyle-related and genetic diseases; requirements for new chemistries for targeted therapies, such as cytotoxins and antibody drug conjugates (ADCs) for cancer; and a rising demand for generics given the off-patenting of drugs.
API exports: Will India gain from a push for supply-chain resilience?
Outsourced APIs currently make up half of global API consumption. This segment is set to grow faster than captive API setups (outsourced APIs are projected to grow at 7% over 2023 to 2030, vs. 4% projected growth for captives). This is driven by cost benefits based on scale, the prevalence of low-cost manufacturing centers in India and China, and the growing demand for generics.
Today, China is a leader in API exports, with a 35% share of the outsourced market primarily playing in high-volume, less complex APIs. However, the API export market is on the cusp of structural shifts as countries look to reduce their reliance on single sources and diversify their supply chains. Developments like the US Biosecure Act aim to further reduce reliance on China, creating opportunities for India to increase its market share.
“India has potential to capture sizeable share from China, subject to timely capex investments by private players enabled by adequate support from the government. The government additionally needs to drive funding for universities and nurture talent in basic sciences to help realize India’s full potential.”
Based on our estimates, China’s portion of the outsourced market is expected to decline by approximately 10 percentage points from 2023 to 2030, with its share likely to stabilize at around 15% by 2047. India stands to gain about 20% to 30% of the share lost by China in the short term due to its lower labor and manufacturing costs, higher service levels, and highly skilled personnel. This gain is contingent, however, on India setting up adequate infrastructure. If it makes those investments, India can reach $12 billion in API exports by 2030, and $80 billion to $90 billion by 2047— matching China’s expected penetration by 2047.
This will require the private sector to develop expertise in innovative chemistries (such as fermentation and high-potency APIs) and drive backward integration for critical KSMs. Integrating the specialty chemical industry with quality standards, technology, and PLI support is crucial. Encouragingly, many specialty chemical firms are already investing in pharma intermediates, but efforts must scale up for India to achieve self-sufficiency.
Generic formulations: Rise of the specialty business and the making of many “home” markets
The global generic formulations market is valued at $460 billion and is projected to grow at 8% p.a. to reach $790 billion by 2030. Indian formulations exports are currently valued at $19 billion. The outlook for India’s exports will not only depend on increasing the volume of generic formulations but also upgrading their value. Enhanced value can potentially come from providing specialty generics in more complex forms like soft gel capsules, inhalants, injectables, and ophthalmics, all of which demand a higher premium.
While specialty generics have a smaller market share, this segment is expected to grow faster than commodity generics (at 12% p.a., vs. 7% for commodity generics). This is driven by the increased demand for more cost-effective drugs for chronic diseases such as cancer and multiple sclerosis, and by efforts to increase patient adherence to prescription plans using novel drug delivery systems. Injectables and inhalants are expected to be the top two modalities (after orals) among the more than 1,000 chemical patent expiries that are anticipated over the next seven to eight years. Specialty generics are difficult to manufacture, are subject to more stringent regulatory requirements, and often need clinical trials, and thus have a higher risk quotient. However, they command higher prices and they offer attractive margins to manufacturers, so many Indian companies have been pursuing this market to gain some pricing leverage.
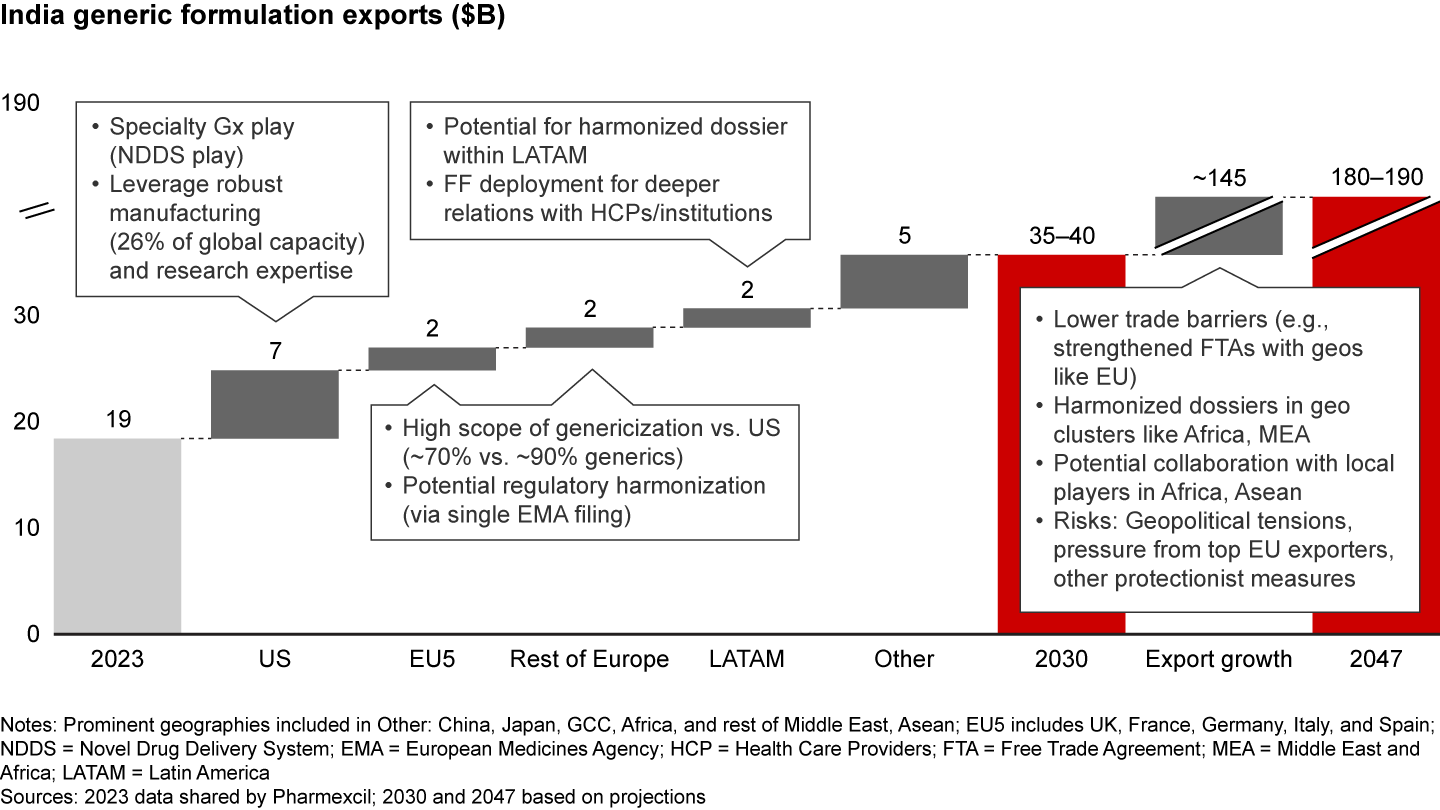
A geographic cluster approach (e.g., US, EU5, LATAM) is crucial to identify where India can increase generics exports. By aggregating these efforts, India is poised to double its generic exports to $35 billion to $40 billion by 2030, and with targeted efforts within each geographic cluster, could reach $180 billion to $190 billion by 2047 (see Figure 9).

To drive growth in commodity generics, which currently dominate formulation exports, private players must secure first-mover advantage, uphold stringent quality standards, and embrace automation to boost efficiency and capital productivity. However, for India to fully realize its potential in formulations, it will require specialty generics to grow equally. There are a few key imperatives for the private sector to turbocharge the growth for specialty generics:
- Developing competencies to conduct clinical trials for specialty generics: Companies should invest proactively in conducting clinical trials and accelerating product development, especially with the 505(b)(2) regulatory pathway in place, which offers faster timelines and reduced risk compared to the conventional NDA route.
- Establishing an on-ground presence in international markets: Indian companies can expand their presence in new geographies by deploying either a field force or adopting a distributor-led model, as applicable. A few Indian players have already deployed a field force in Africa to expand their presence.
- Building skills in M&A and business development: Most companies are learning the specialty generics business through the acquisition of existing brands. Many are also tying up with companies to share risks and build front-end capabilities.
As mentioned earlier in the section on key megatrends, digitalization is set to be a key growth driver for APIs and formulations, with companies like GSK and Sanofi using Industry 4.0 to improve efficiency. In India, Cipla and Dr. Reddy’s lead in implementing these technologies. However, India’s pharmaceutical exports face quality challenges, with approximately 89% of site inspections between 2015 and 2024 resulting in NAI or VAI (Voluntary Action Indicated) outcomes, compared to 96% to 98% in Japan and Europe. While the industry is addressing this, stronger technology adoption is essential to ensuring consistent quality standards.
Alongside the key imperatives detailed for the private sector, strong policy support from the government is also important to ensure that Indian pharmaceutical players get the regulatory support and investment incentives that will help them grow their exports. The recent adoption of Schedule M, akin to WHO GMP standards, is a welcome start. India needs to continue efforts to become part of PIC/S and be counted among the regulatory bodies with best-in-class standards. Specifically, government entities should consider taking the following steps:
- Implementing progressive regulations and policies: Tax incentives and subsidies, such as the inclusion of R&D costs in allowable tax deductions, will be key drivers for future R&D activities, especially when it comes to developing innovative API chemistries and complex generic formulations. The government could also include outsourced clinical trial costs as part of R&D activities, since these are increasingly going to be larger than internal R&D spending. More work could also be done to simplify regulatory processes, such as by allowing top pharmaceutical companies to perform self-assessments as part of regulatory approvals.
- Offering market access assistance to private companies: Governments are increasingly supporting exporters by offering guidance on market landscapes and the latest market developments and providing promotional assistance. Germany’s “Health Made in Germany” program helps pharma companies through trade fairs and conferences. Indian companies can also secure preferential market entry into regions like the EU through trade agreements and market entry guidance. While Pharmexcil’s initiatives like Japan’s 2023 roadshows were helpful, there is potential to expand these efforts.
- Setting up dedicated pharmaceutical and bulk drug parks to attract investments in India: Providing incentives to establish consolidated infrastructure dedicated to pharma R&D and production can lower capital expenditures (capex) and operating expenses (opex) and help firms build a competitive advantage. Larger parks—like China’s special economic zones (SEZs)—are 10 to 15 times the size of India’s parks and achieve higher operating efficiencies and lower overheads. India has established common infrastructure facilities with a $350 million outlay for three bulk drug parks in Gujarat, Himachal Pradesh, and Andhra Pradesh; however, these efforts need significant scaling and need to be supported by centralizing energy supply, setting up waste treatment in advance, and improving road connectivity.
It is critical that private companies and governmental and trade bodies come together to drive an increase in volume and upgrade the value of exports.
Biosimilars: Catch-up and leapfrog
Biosimilars, valued at about $30 billion, make up about 7% of the $410 billion global biopharmaceutical market. The biosimilars market is expected to grow at 20% p.a. and reach the $100 billion mark by 2030. This growth will be driven by multiple factors including increasing share of biologics over small molecules, rising incidence of chronic diseases treated by biosimilars, ongoing regulatory changes, growing demand for cost-effective therapies, and an impending patent cliff for multiple biologics. Over the next seven years, 130 drug patents valued at a total of $180 billion are expected to expire, creating a sizeable opportunity for Indian companies.
Indian pharma firms currently hold less than 5% of the global biosimilars market, but top companies are investing in R&D and expanding their pipelines with over 40 new products. Their plans to add close to 100 kiloliters of capacity over the next three to four years could increase India’s share of global capacity from approximately 7% currently. Public initiatives like the National Biopharma Mission, PLI programs, and BIRAC-led initiatives are positioning India well to capitalize on biopharma growth. For example, Telangana aims to triple its life sciences industry value by 2030 with the expansion of Genome Valley by 300 acres being a key initiative.
“India has played a vital in role in enabling access to low-cost generics globally. As the treatment paradigm shifts towards biologics, India has the potential to transform healthcare through high quality, affordable innovation and claim its rightful place as a global thought leader. This transformation is key to realizing our national ambition of a ‘Viksit Bharat’ as focus shifts from cost arbitrage to value creation.”
Full potential for Indian biosimilars exports and key imperatives
The Indian biosimilars exports market is expected to follow a trend like formulations with about an eight-year lag. Its current value of $0.8 billion is expected to increase fivefold, to $4.2 billion, by 2030, when Indian biosimilars exports could comprise 4% of the global market, equivalent to formulations exports in 2022 (see Figure 10). By 2047, the value of Indian biosimilars is expected to reach $30 billion to $35 billion. Growth will be driven by global trends such as simplification of biosimilars pathways in the US, including interchangeability and bioequivalence. The Biosecure Act will also have a positive impact. However, India lags China and South Korea in this arena and needs to invest strategically to succeed in this market.
“Interchangeability has come in and bioequivalence will also come in soon, removing the need for clinical trials. This will streamline capital allocation and drive biosimilars growth. We foresee a future with 20–30 major global players, including 4–5 large-scale, credible Indian companies.”

To realize the full potential for biosimilars exports, private firms will have to play a key role. Some of the imperatives include:
- Choosing between multiple routes to market for entering new geos: Companies must carefully choose between organic growth, in-licensing, partnerships, or M&A. Indian companies have partnered with MNCs and local players to expand their presence in regulated and emerging markets. These partnerships help share clinical trial risks and build trust with healthcare providers to encourage biosimilars adoption.
- Devising innovative commercial approaches to boost market penetration: Companies have followed diverse approaches. For instance, AMJEVITA has a tiered pricing structure targeting PBMs and insurers separately, while Coherus Biosciences offers YUSIMRY at a flat 35% discount through partnerships.
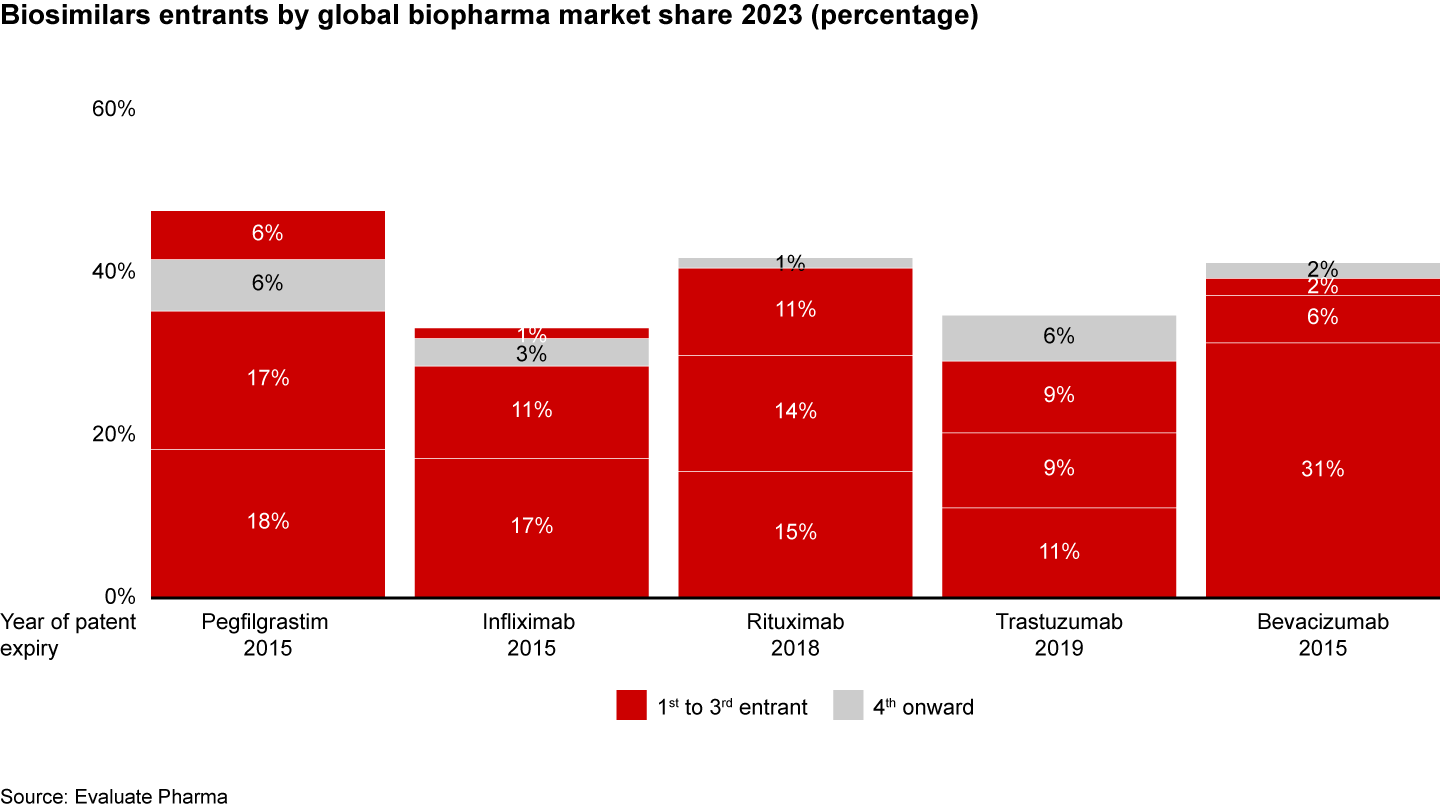
- Accelerating drug development: Being among the first three biosimilars entrants is critical to capitalizing on innovator patent expiries. For instance, the first three biosimilars for Pegfilgrastim, Infliximab, and Rituximab captured 30% to 40% of their respective biopharma markets (see Figure 11).
- Adapting to varying biosimilars approval pathways: Navigating biosimilars approvals requires prior knowledge of local requirements and adapting strategies proactively. While countries like Australia, South Africa, and Brazil accept multi-country clinical trial data, others, such as Russia and Mexico, are more stringent and mandate local trials. In the case of Russia, however, global clinical trials data are accepted, provided the trials include Russian patients. Prior knowledge of geo-wise nuances will help Indian players streamline approvals.
- Evaluating CDMO in biopharma: For firms to realize the full potential of biopharma opportunities, they need to invest in CDMO, so they can build out their capabilities while waiting for clinical trial outcomes.

All these efforts must be complemented by government initiatives to establish biopharma parks, lower start-up costs, offer R&D incentives, encourage skill upgrades, and encourage partnerships. Singapore’s Biopolis Park—launched in 2003 to grow the biomedical industry—provides infrastructure, funding, and tax incentives, and attracts talent through scholarships. South Korea also serves as a helpful case study. It has 18 biopharma clusters with plans to add two more, attracted global companies, and enabled joint R&D among Korean companies, medical institutions, and research institutes. A joint incubator with global companies and the Korean Trade-Investment Promotion Agency (KOTRA) supports local bio-start-ups with funding.
Vaccines: India as a global leader in disease prevention
Vaccines are vital to global public health, saving millions of lives every year. The global vaccines market, already sizeable at $80 billion, is poised for steady growth of 4% p.a. between 2023 and 2030. The market experienced a sharp surge from $41 billion in 2020 to $144 billion in 2021, driven by Covid-19 vaccines. However, the proportion of Covid-19 vaccines as a share of the market is expected to decline gradually, from 70% in 2021 to less than 20% in 2030, as vaccination rates and natural immunity rise. The demand for non-Covid vaccines is expected to grow at 7% p.a. between 2023 and 2030.
HICs like the US, Canada, and Europe dominate the vaccine market, accounting for 65% by value. Historically, vaccine growth in HICs has been marginally slower than the whole market (18% p.a. vs. 19% p.a. from 2019 to 2023) due to high immunization coverage in developed economies, which leaves limited room for market expansion. In contrast, MICs and low-income countries (LICs) such as China, Argentina, and African nations have experienced faster growth (22% p.a.). This trend is expected to continue as new vaccines are integrated into national immunization programs and healthcare access in these regions continues to improve.
Vaccine exports: India’s journey from volume to value
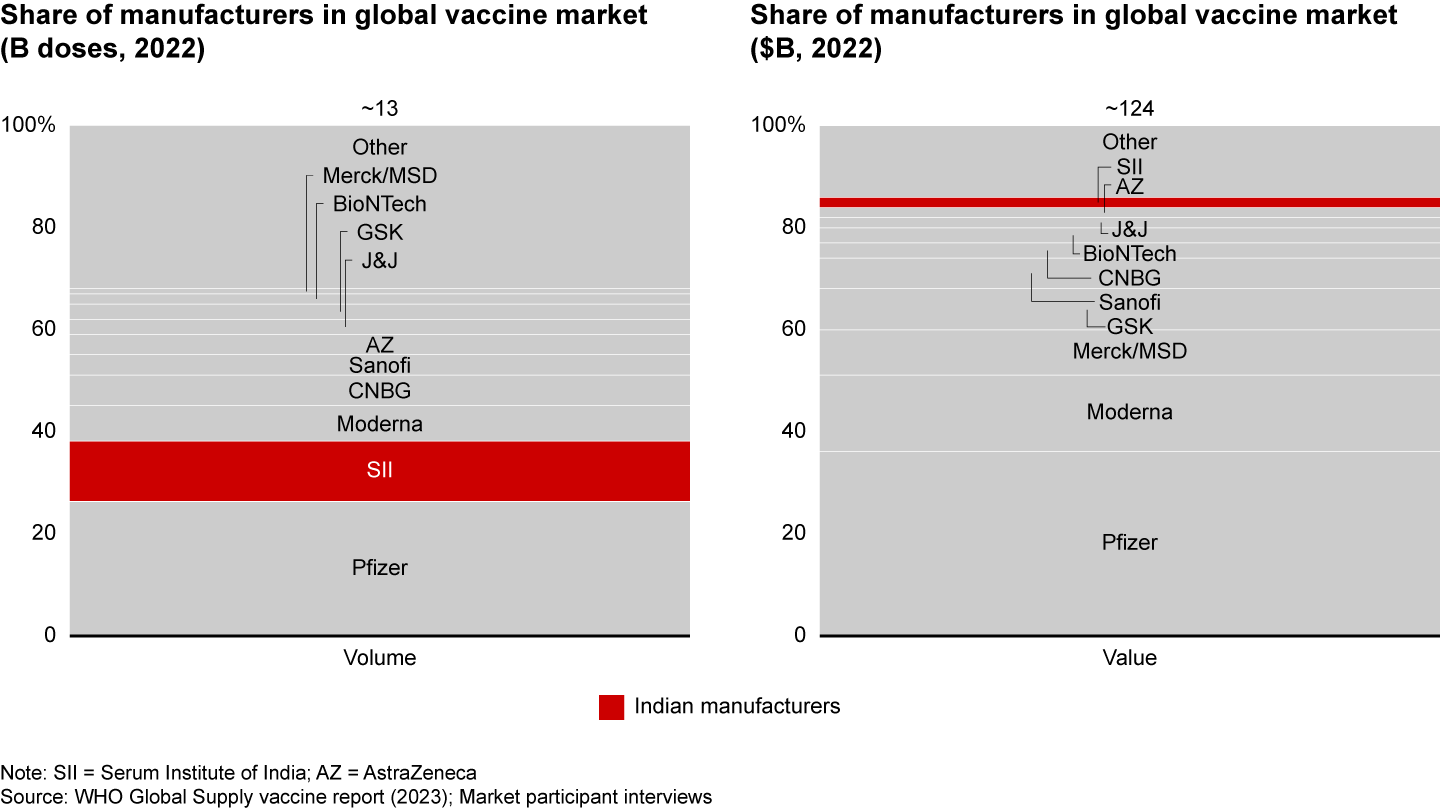
When it comes to vaccine exports by volume, India fares well in the global market (including Covid-19) with respect to volume, with Serum Institute holding 12% market share, second only to Pfizer. However, India lags by value, with Serum ranked 10th and holding just 2% share (see Figure 12). This disparity is due to India’s focus on affordable vaccines for LICs via UNICEF and its challenges in penetrating high-income markets, where its reputation as a low-cost producer affects trust.

“Developed countries exhibit hesitancy towards vaccines from the developing world. These markets are typically dominated by MNCs as they are usually the innovators and have a head start (due to patent protections) in establishing trust in the market. Vaccine similars are late entrants to market and therefore, do not have enough time to garner market confidence.”
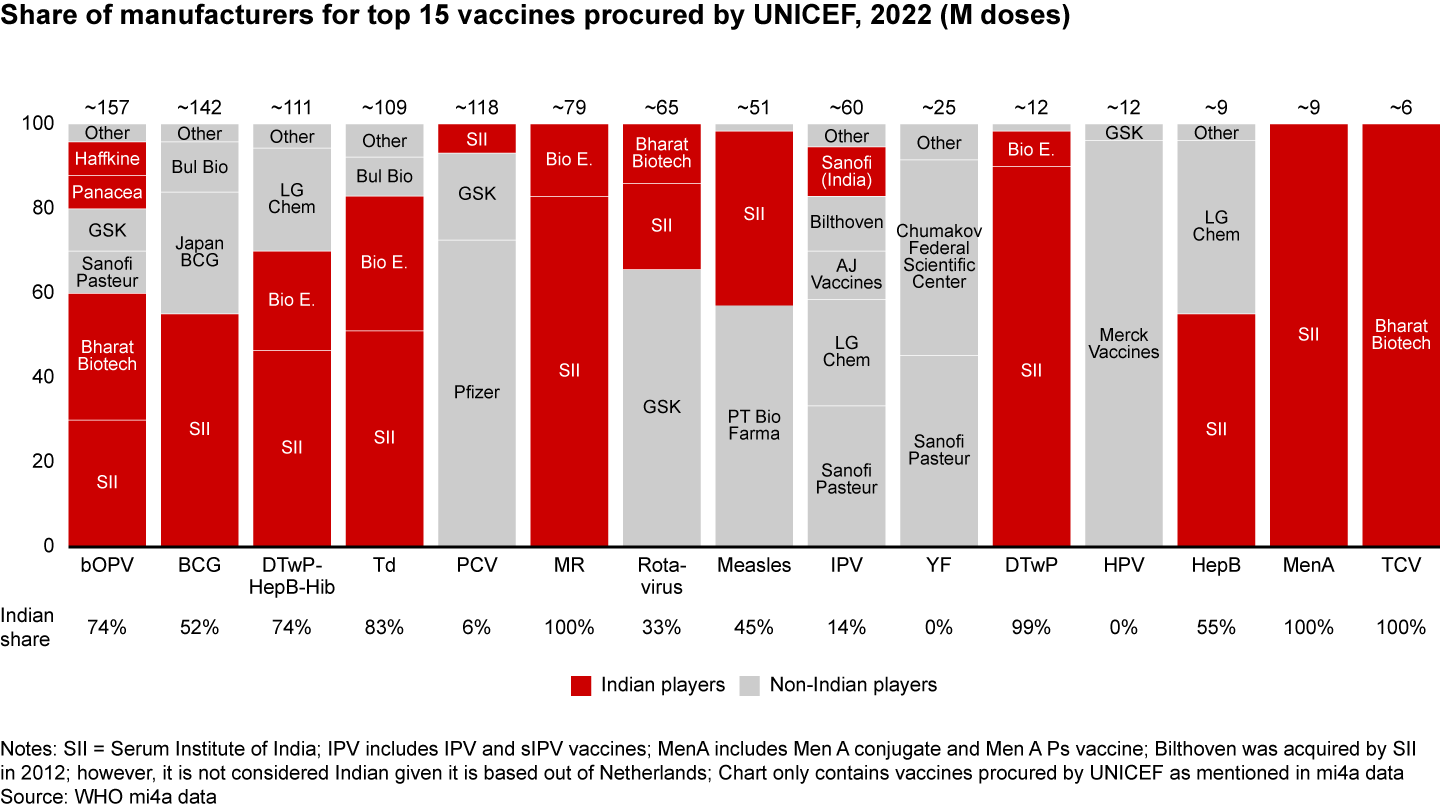
India has been UNICEF’s largest vaccine supplier for the past six to seven years, contributing 55% to 60% of total volume procured, showcasing its expertise in pediatric vaccines. Indian companies like Serum Institute, Bharat Biotech, and Biological E hold significant shares in key vaccines (see Figure 13). However, India has room to grow in critical vaccines like Pneumococcal Conjugate (PCV), Inactivated Polio (IPV), Rotavirus, Yellow Fever, and Human Papillomavirus (HPV), where it currently lags.

Despite its strong position in the public vaccine market, India’s share of the private vaccine market remains minimal. Here, market share is dominated by MNCs such as Merck, Pfizer, GSK, and Sanofi. Indian players are nonexistent in HIC private markets and have less than 2% share of MIC private markets.
Full potential for Indian vaccine exports and key imperatives
There is an opportunity for India to increase its export penetration in the global vaccine market from 1.5% currently, to 3% by 2030, and to 8% by 2047. If India increases its penetration of MIC/LIC markets in the near term (from 3.3% currently, to 7.5% by 2030, and stabilizing at about 15% by 2047), and pursues the HIC market in the long term (potentially reaching 3% by 2047 through targeted interventions), these goals are achievable. Through these strategies, the value of Indian vaccine exports could grow from $1.1 billion currently, to $3 billion by 2030, and to $24 billion to $25 billion by 2047.
“India should focus on continuing to expand its MIC/LIC footprint in the short term, while focusing on penetrating HICs as a medium-term goal. Building trust through Key Opinion Leaders and demonstrating quality will enhance India’s brand and pricing power, particularly in MICs and HICs. But in the long-term, there is no alternative to moving up the value chain in the innovation space to shape its rightful place in global preventive health space. Aligning regulatory standards and practices with the US and EU will further ease India’s entry into developed economies.”
To achieve this, Indian manufacturers should focus on these key strategies:
- Targeting the right geographies and products: There is potential for companies to increase their footprints in key vaccine segments such as PCV, seasonal flu, and hepatitis, where India’s current share is limited.
- Targeting MICs in the near term: To succeed in MIC public markets, Indian manufacturers should form partnerships with local pharma companies and encourage advocacy from key opinion leaders. Entry strategies should adapt to each country’s dynamics. In MIC private markets, vaccines like HPV, Influenza, Rabies, PCV, and Hexa are attractive opportunities, with India having the expertise, but currently having limited to no market share.
- Aiming for expansion into HICs in the medium to longer term: India should focus on high-demand vaccines such as influenza, PCV, HPV, meningococcal, measles-mumps combinations, Diptheria, Tetanus, and acellular Pertussis (DTaP), and Hexa, where firms already have capabilities. However, success in HICs will require significant investment in clinical trials, regulatory approvals, robust manufacturing processes, and go-to-market (GTM) capabilities.
- Leveraging partnerships for technology transfers: India is currently under-indexed in emerging vaccine technologies such as Multiple Antigen Presenting Systems (MAPS) and Generalized Modules for Membrane Antigens (GMMA), among others. To stay competitive, Indian companies should partner with research institutions and MNCs for technology transfers.
- Expanding presence in the adult vaccine market: India’s vaccine exports are largely limited to pediatric vaccines, with companies only recently making forays into adult vaccines. It is imperative for Indian firms to increasingly expand into adult vaccines—like Shingles and Respiratory Syncytial Virus (RSV)—and adopt emerging technologies, such as long-acting antibodies and mRNA, to capture a larger share of the global market.
- Building CDMO plays to enter new geographies: Indian companies can explore becoming contract manufacturing partners for global players. For instance, Biological E. entered a partnership with Takeda for production of Qdenga, a dengue vaccine, which targeted “India for India” and “India for the World” strategies.
To enhance India’s vaccine export potential, private sector initiatives must be bolstered by comprehensive and forward-looking government measures, including:
- Establishing vaccine-focused hubs: India has several biotechnology clusters but lacks a dedicated focus on vaccines. Globally, countries are investing in vaccine research hubs, like Vax-Hub, a collaboration between University College London and Oxford. India must expand its biotech centers and establish vaccine research parks to stay competitive globally.
- Easing regulatory pathways and providing financial support: India’s current multi-tier regulatory processes should be streamlined to reduce time to market, with flexible pathways for established players. For instance, simplifying Form 29 No Objection Certificate (NOC) and Review Committee on Genetic Manipulation (RCGM) approvals could shorten approval timelines from 30 to 56 months to 14 to 26 months. Manufacturers should also be allowed to stockpile vaccines at their own risk, with licenses only required for commercialization. Finally, considering the global trend for incentivizing domestic manufacturing (such as via the African Vaccine Manufacturing Accelerator), India should support its own vaccine producers with funding for capex investments abroad, especially in Africa, to counter these threats.
- Developing talent specific to vaccinology and immunology: India should prioritize the development of talent specific to vaccinology and immunology to cater to the rising demand for skilled professionals as the industry continues to expand.
- Encouraging R&D for new vaccine technologies and vaccine-preventable diseases: The government should set up funds to encourage Indian firms to invest in new vaccine technologies like mRNA and GMMA, and in research for targeting new pathogens and creating a new set of vaccines for preventable diseases. For instance, India can focus R&D on diseases caused by pathogens like HIV-1 and Norovirus, which are currently not preventable by vaccines.
With strategic expansion, technological innovation, and robust government support, India is on track to solidify its position in the global vaccine market, not only as a volume-based leader but also as a value-based leader.
Innovative products (NCEs/NBEs): The last frontier
Patented drugs currently make up the largest segment of the global pharmaceutical market. Valued at $800 billion, this segment is expected to grow at 8% p.a. to $1.4 trillion by 2030. One growth driver is the rising prevalence of chronic diseases; cancer cases globally are expected to grow 1.8 times between 2022 and 2050, and the number of patients with diabetes is expected to jump 1.5 times between 2022 and 2045. Increasing investments in R&D and the adoption of innovative therapies across the world will also be contributing factors.
Biologics are expected to make up the bulk of this growth. Currently constituting 45% of the market, biologics are expected to grow at 10% p.a. to make up 55% of the market by 2030. The rising acceptance of biological therapies hints at this future growth; 25 novel oncology-focused biologics were launched in 2023 alone. In 2023, 15 of the top 20 drugs were biologics, whereas 10 years ago, this category of drugs only captured 10 out of the top 20 positions.
The Indian pharmaceutical industry, though nascent when it comes to innovative products, has shown promise, with India’s top 10 pharmaceutical firms reporting more than 40 new products in the NCE/NBE pipeline. Three models currently exist for Indian companies to rise to prominence in innovative product exports:
- In-licensing/acquisition of already-developed products from global players: This involves getting distribution rights for patented products developed by global pharmaceutical companies. Indian companies such as Sun Pharma and Intas have successfully commercialized innovative products like Ilumya (Merck) and Serplulimab (Henlius), which were developed abroad. This model allows players to diversify their portfolio and enter the innovative space without the need for extensive R&D investments. This strategy works just as well for global markets as for the Indian market.
- Out-licensing of pipeline assets developed in India: Indian companies with promising early-stage products (usually pre-clinical or in Phase 1/Phase 2 trials) often out-license these assets to global players for further development and commercialization due to limited resources. Monetization models include milestone payments, royalties, or a combination of both, all of which allow for faster pipeline monetization and reduced clinical trial risk. This also leverages the partners’ marketing reach. Six out of the top ten Indian pharmaceutical players (by revenue) currently out-license their NCE/NBE assets.
- End-to-end discovery, development, and commercialization of products: Though not yet fully explored in India, this model keeps the complete life cycle of drug development, from discovery to commercialization, within India’s borders. While there are currently few marquee examples (Saroglitazar is one), Indian companies are planning to invest heavily in building the capabilities to create fully in-house-developed innovative products. This is the source of the largest untapped potential for India to become a true global pharma powerhouse.
“We are optimistic of one Indian company eventually developing an innovative product end to end in the near future, setting in place a virtuous cycle that inspires others to follow suit.”
Full potential for Indian innovative exports and key imperatives to be a sizeable player
Success in innovative products will require Indian companies to proactively take some key steps:
- Prioritizing R&D on rare diseases: Orphan drugs are often used for the treatment of rare diseases. The orphan drug market is attractive and fast-growing; it’s expected to grow at 10% p.a. from 2023 to 2028, with oncology emerging as the most attractive segment (contributing 40% of total revenue in the orphan drugs category). This makes it critical for innovators to build capabilities for the development of orphan drugs within India.
- Ramping up R&D investments: There is currently an 18-percentage-point gap between the R&D spending of top Indian companies, as a percentage of revenue, and the R&D investments of the top 10 global pharmaceutical companies. However, Indian companies have robust plans to invest in R&D going forward. Ramping up R&D investments and focusing on large and fast-growing therapeutic areas (TAs) like oncology will drive growth. Further, Indian companies can follow in China’s footsteps, adopting a fast follower approach of researching new molecules based on successful preclinical or early-stage clinical trial data to get a head start on developing innovative products.
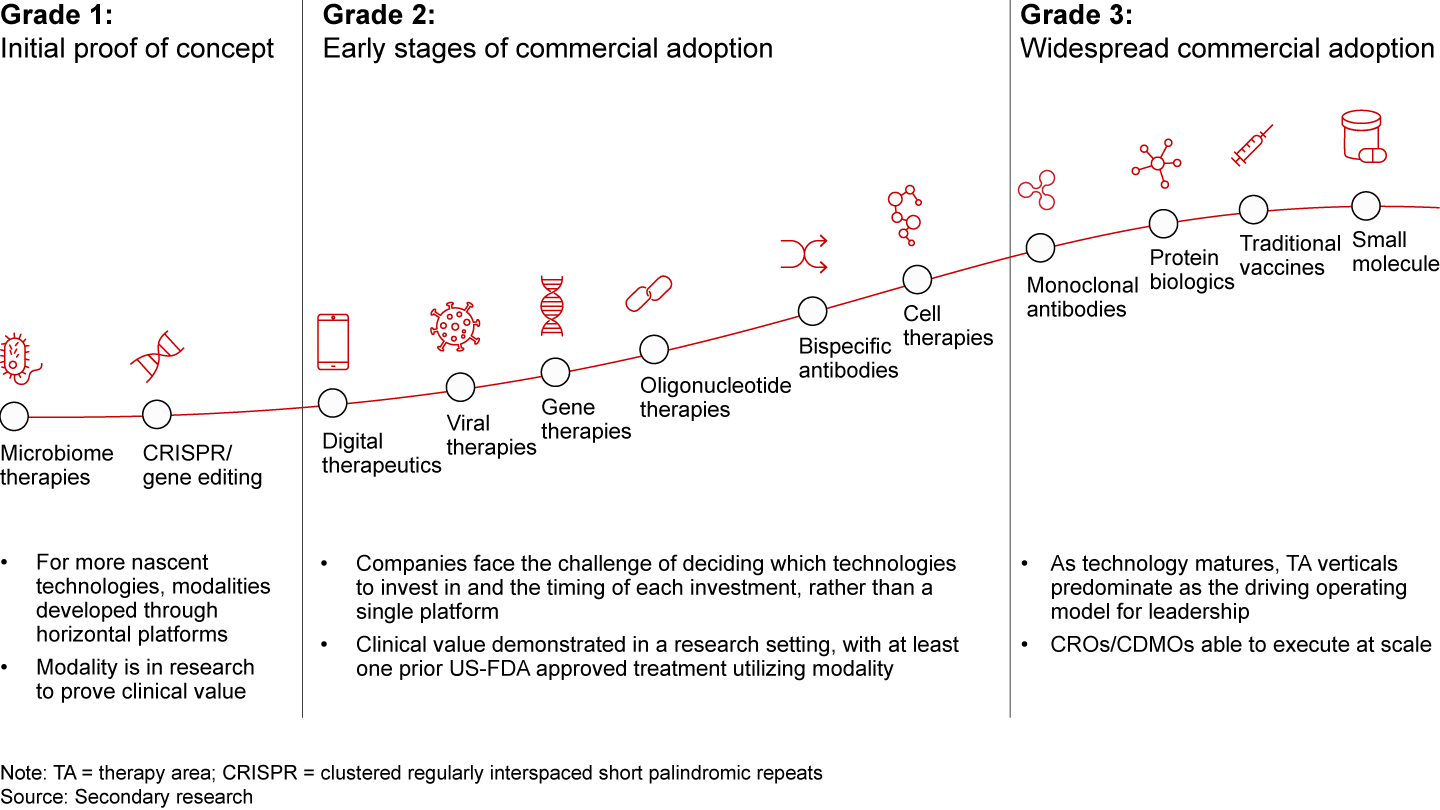
- Developing expertise in new modalities: Pharmaceutical technology evolves in three stages, each needing a unique approach. Grade 1 is typically driven by academic research focused on proving clinical value; Grade 2 involves start-ups experimenting with promising technologies; and Grade 3 sees large-scale industry adoption. Indian companies should prioritize emerging technologies in early commercial stages (Grade 2), like cell and gene therapies, which are expected to dominate future drug pipelines (see Figure 14).
- Integrating AI/ML in drug discovery: Artificial intelligence and machine learning (AI/ML) technologies are transforming drug discovery by improving the efficiency of target identification, compound screening, and lead optimization. As a result, the number of AI-discovered molecules has grown by more than 40% p.a. between 2018 and 2021. It is thus imperative for Indian companies to either invest in building in-house AI capabilities or collaborate with global technology vendors to stay competitive. Indian start-ups are increasingly leveraging AI-based technologies for advanced drug discovery; however, there is tremendous potential to scale this up.
- Collaborating with Big Pharma, bio-techs and academia: Partnerships between Indian companies and global pharma, biotech, or academic institutions can accelerate innovation. For instance, Pfizer’s collaboration with IIT Delhi supports early-stage research and IP development. Globally, models like J&J Innovation’s JLABS, incubating over 1,000 start-ups, and Singapore’s Agency of Science Technology & Research (A-STAR), which fosters collaboration between pharma and research institutions, offer strong examples of such partnerships.

“We are targeting to increase our R&D investments and expect to take it from ~7% to ~9% of revenue, over the next 3–5 years.”
Government also has an important role to play. It can encourage the participation of academia, drive subsidies to encourage R&D, enact favorable IP laws, offer funding to encourage companies to develop more innovative products, and provide clear and seamless regulatory pathways. A conducive funding environment from incubators and VCs will also help grow biotechs and allow Indian firms to be competitive internationally. US biotech companies secured $57 billion in funding in 2023 alone, while Indian biotech firms raised only $5 billion over a decade (2013 to 2022), highlighting a significant funding gap. Government initiatives like the National Biopharma Mission, research-linked incentive (RLI) schemes, and support from BIRAC, which has funded over 1,800 companies, are promising, but further investments are required to expand India’s biotech sector.
“The government needs to establish a fund to partially finance patients treated with innovative drugs, driving affordability, and limiting downside for companies. This will dramatically spur innovation and encourage firms to take more risks.”
“Our regulatory framework must prioritize expedited approvals and adopt a consultative approach. Empowering regulatory bodies with the right talent and scientific expertise is essential to drive an innovation ecosystem.”
Addressing these imperatives can transform India from a generics manufacturer to a hub for innovative drug discovery and development. By 2030, innovative exports could reach $2 billion, driven by in-licensing global products and out-licensing Indian pipeline assets. With end-to-end in-house product development, exports could grow much more, to $13 billion to $15 billion by 2047. However, even these estimates are conservative, and if a few things go India’s way, there is a clear potential for the innovative segment to become a sizeable chunk of the India pharma exports basket.
CDMO/CRO: Leadership in services leading to leadership in products
As global pharma increasingly turns to outsourcing, the role of CROs and CDMOs has become more critical than ever. The global pharmaceutical outsourcing market can be categorized in three key segments: discovery, development, and commercialization. Discovery services encompass early-stage activities like target identification, validation, hit and lead generation, safety testing, and lead optimization. Development and commercialization phases cover a wide array of functions, from preclinical research and clinical trial management to regulatory approval, manufacturing, sales, marketing, and distribution. While CROs primarily focus on the discovery phase, CDMOs are responsible for development and commercialization services.
The global CDMO and CRO market currently stands at $187 billion and is projected to grow at 10% p.a. to reach $355 billion by 2030. Its primary growth drivers are a robust global R&D pipeline of more than 21,000 drugs, the integration of advanced technologies like AI/ML to accelerate drug discovery and development, and remote monitoring tools for improving supply chain traceability. Additionally, margin pressures from tighter drug pricing and reimbursement rules in developed economies like Germany may drive increased outsourcing to more cost-effective markets like India.
CDMO/CRO exports: Making India a hub for pharmaceutical outsourcing
As highlighted in the megatrends section, CDMOs and CROs are leading India’s push toward innovation, often by out-licensing their own IP-driven products to front-end partners. By expanding their offerings from generics to value-added, innovative products, Indian CDMO and CRO players can build expertise, enabling Indian pharmaceutical companies to develop their own innovative products that will help fuel the transition from volume to value.
Historically, Indian CDMO and CRO firms have primarily focused on small molecules. India’s CRO market doubled between 2019 and 2023, reaching a total value of $2.5 billion. This impressive growth implies a growth rate of 18% to 20% p.a., as compared to global market growth of 11% to 13% p.a. during the same period. India’s growth trajectory is expected to continue, driven by its large, highly skilled talent pool (with approximately 2 million science, engineering, and tech graduates every year) and a growing ecosystem of tech start-ups leveraging AI-based drug discovery. Additionally, continued improvements in India’s IP-protection regime will be crucial in attracting outsourcing from global pharmaceutical companies. For context, India’s score on the International IP Index has already risen from 25% in 2017 to 39% in 2024.
India’s CDMO market also doubled since 2019, reaching $20 billion in 2023. Future growth of this market will be largely driven by the push by developed economies to diversify their supply chains and reduce reliance on single sources like China. A key catalyst for this shift is the US Biosecure Act, which restricts partnerships with major Chinese biotech firms for critical pharmaceutical ingredients. Indian CDMOs offer a compelling alternative, with better quality, lower costs, and superior service levels, making them increasingly attractive to global pharmaceutical companies.
Full potential for Indian CDMO and CRO exports and key imperatives
Given these dynamics, India’s CRO exports as a share of the global CRO market is set to expand from 5% to 6% currently, to 7% to 8% by 2030, to 10% by 2047, and eventually reaching $20 billion. This will primarily be driven by small molecules, but there is also the potential to establish some presence in large molecules. India’s CDMO exports as a share of the global CDMO market is also set to increase from 10% to 11% currently, to 13% to 15% by 2030, to 15% to 17% by 2047, and eventually reaching $130 billion. This will involve leapfrogging China by 2030 in small molecule CDMO exports and matching China’s current share in large molecule CDMO exports by 2047.
“The future of Indian CDMOs is bright, with sustained growth bolstered by government support to manufacture top APIs/KSMs. Indian players are well-poised to build the size and scale needed to compete with Chinese counterparts globally.”
Collectively, India’s CDMO and CRO exports are projected to increase from $18 billion in 2023 to $44 billion by 2030 and to $150 billion to $155 billion by 2047. To get there, it is imperative that private sector players lead the way through targeted initiatives, such as:
- Demonstrating sufficient installed capacity: Approaches for building capacity include using excess manufacturing for CDMOs, expanding based on client letters of intent, and utilizing investor funding. Indian companies can also secure contracts by leveraging their credibility in support functions, as seen with Big Pharma outsourcing innovation and digital services to India.
- Strengthening AI/ML capabilities: AI/ML can streamline drug discovery and preclinical phases, optimize development processes, and reduce time to market.
- Expanding capabilities in specialty forms and complex technologies: Firms should invest in fast-growing technologies like Peptides, High-potency APIs, and ADCs to capture a larger share of the market.
- Developing a scaled biologics play: Companies should also look to invest in biologics-focused discovery, development, and manufacturing capabilities. Leading global players like Samsung Biologics and WuXi Biologics have set benchmarks by establishing large-scale biologics and biosimilars capacities, and they currently have robust expansion plans.
To support the private sector, it is also essential that the government take proactive initiatives, such as:
- Improving GMP adherence: Regulators can increase surveillance of manufacturing facilities and enforce stricter regulations, including revised Schedule M, to align with global GMPs and ensure strict quality enforcement.
- Promoting clinical trial activities in India: India’s large patient pool, improving regulatory environment, and skilled workforce make it an attractive destination for global clinical trials. To fully capitalize on this potential, India should lift restrictions by the Central Drugs Standard Control Organization (CDSCO) on conducting first-in-human (FIH) studies for drugs discovered internationally. Additionally, expanding initiatives like the RLI scheme, with dedicated funds for clinical trials, can significantly boost India’s clinical research landscape.
- Supporting talent growth in the pharmaceutical sector: China’s Thousand Talents Plan, which attracts foreign-educated Chinese scientists through research funding and a collaborative ecosystem, is a strong model for India to follow to build its talent pipeline. Additionally, forging industry collaborations with the National Institutes of Pharmaceutical Education & Research (NIPERs) can nurture market-ready talent.
By aligning government initiatives with the private sector’s strategic actions, India can solidify its position as a critical hub for pharmaceutical outsourcing.
“CDMOs provide an attractive opportunity for India—however, to fully realize this, we need to have consistent and predictable regulations along with a culture of quality, safety, sustainability coupled with a highly skilled talent pool.”
Key recommendations for the government
Case study: China’s ascent to leading pharma exporter
China’s ascent offers some critical lessons for India’s policymakers and pharma-industry leaders. Beginning with its economic reforms of 1978, China strategically leveraged SEZs and industrial parks to attract foreign investment. In the 1990s, China expanded SEZs to 14 additional port cities and launched new cooperation zones (such as the China-Singapore cooperation zone), offering lower corporate taxes, low-cost land, and minimal custom duties, catalyzing industrial growth. Massive infrastructure investments in railways, expressways, and inland waterways boosted logistics for exports.
China’s rise in the global pharmaceuticals market is largely driven by its API exports. In the early 2000s, China seized the opportunity presented by global pharma companies outsourcing API production due to associated environmental risks. China responded with policies that nurtured API manufacturing. From 2001 to 2015, China’s five-year plans emphasized API self-sufficiency and drug security through creation of drug parks (like China Medical City in Jiangsu province) with common facilities, common effluent treatment plants, and uninterrupted power supply. To further encourage API exports, China provided financial supports through tax incentives, exemptions, and long-term low-cost loans to exporters of bulk drugs. These measures helped grow China’s API exports nearly 10-fold over 20 years.
Regulatory reforms, such as 2005’s Drug Registration Measures and 2010’s GMP reforms, aligned Chinese standards with international benchmarks, enhancing global credibility. Joining the International Council for Harmonization of Technical Requirements of Pharmaceuticals for Human Use (ICH) in 2017 cemented China’s regulatory reputation. China also fostered R&D through programs like the National Science and Technology Major Project for Drug Innovation in 2008, which supported basic research on chemical compounds, preclinical pharmacological research, and clinical studies. With policies like the Thousand Talents Plan to attract non-resident Chinese scientists, China focused on strengthening its talent base to aid innovation.
Building on its R&D capabilities, China is beginning to transition to biosimilars and innovative products through its “Made in China” policy, which identifies biopharmaceuticals as a key development sector. The expanding National Reimbursement Drugs List underscores growing support for these drugs. Additionally, the government’s volume-based procurement program for generics has reduced profitability in the generic sector, pushing manufacturers to shift their focus to advanced products like biologics.
In addition to the segment-specific imperatives highlighted in the sections above, the takeaways from the China case study and from our discussions with industry leaders point to the following recommendations for the government in the short, medium, and long term to enhance India’s pharmaceutical exports:
Short-term recommendations
1. Create a conducive environment for R&D: India has taken a significant step toward boosting R&D by rolling out RLIs in 2023 with a planned financial outlay of $600 million from FY24 to FY28. This initiative covers multiple categories, including NCEs, NBEs, complex generics, biosimilars, medical devices, and orphan drugs, and it will be pivotal to supporting India’s shift from volume to value. Recently, six pioneering start-ups were funded under the program. However, it is imperative that the government ensures timely disbursal of funds and significantly increases the quantum of funding, given the high-risk and long timeframe involved in pharmaceutical research. Government can further incentivize R&D through tax incentives (such as potentially bringing back 200% tax deductions on R&D expenses, tax holidays for innovation-led products, and concessional taxes beyond a defined hurdle rate of R&D spending).
Moreover, clinical trials in India face cumbersome approval processes, including requirements to provide undertaking at the application stage and subject expert committee (SEC) meetings, which prolong timelines by more than four months. Streamlining these regulatory processes is key to fueling innovation.
“While the INR 5,000 Cr allocation as a Research Linked Incentive is a welcome and positive step, the amount needs to be commensurate to the at-scale innovation that India needs. With research outlay of hundreds of millions required for true innovation, we need a multiple of the proposed RLI to make this dream a reality.”
2. Chart a granular view of the global demand landscape with country-specific targeted interventions: As highlighted earlier, NAFTA and Europe are focus areas for generic formulations and biosimilars. Discussions with industry bodies point toward key interventions that India can pursue. Within NAFTA, the US and Canada are attractive and require targeted country-specific action plans by the government. For instance, India can seek an exception in the US on the over 55% domestic component rule for federal imports (exceptions exist for WTO GPA members, of which India is not currently a part). It can further request that Indian-sourced products qualify as coming from a “designated country” under the Trade Agreement Act (TAA), which would make companies more effective in their bids for government procurement contracts. Similarly, in Canada, India can potentially put forth a point during FTA negotiations, regarding patent protection being limited to only Canada. The Canada Supreme Court’s current interpretation allows Canadian patents to have extraterritorial applications, meaning a drug produced with a patented intermediate infringes the patent when the final product is imported and sold in Canada.
In the EU, current regulations require analytical testing of every batch of an imported consignment at the port of destination, with the consignment only allowed to reach the market after satisfactory results. This slows market arrival by two to three months. India may seek a revision in this procedure based on quality checks during domestic inspections, eliminating the need for double testing. Further, most generic players in the EU use the decentralized procedure (DCP) rather than the centralized pathway for marketing authorizations. A major barrier is the six-month-plus wait time for a DCP appointment, which delays filings even when dossiers are ready. Unlike in the US, where ANDAs can be filed immediately, this process hampers timely submissions. Faster DCP appointments would benefit Indian manufacturers and ensure earlier availability of generics in the EU.
While EU guidelines mandate national approvals within 30 days of DCP closure, some countries delay by nine to fifteen months, further complicating product launches. This is especially challenging for low-volume, high-value drugs like oncology and hormone products, where manufacturing depends on securing approvals in all countries.
Within the UK specifically, India can push for “Day One” launch immediately after the patent expiry for the formulation. Moreover, with the NHS controlling nearly all pharma procurement, ensuring non-discrimination in contracts is crucial and can be addressed in ongoing FTA discussions. Additionally, there is a need to pursue pharmaceutical-specific MRAs to address non-tariff barriers in key markets by harmonizing compliance standards, which would ensure easier market access for Indian exporters.
3. Drive high-impact initiatives to encourage trade: There is the potential to enhance the attractiveness of Remission of Duties and Taxes on Export Products (RoDTEP), which was designed to reimburse taxes and duties that are not refunded through other means, like municipal taxes or cess on transport. Currently, the reimbursement rate for most pharmaceutical items is 0.5% to 1.2%. If this percentage were increased, it would help level the playing field for Indian exporters and make them more competitive globally.
4. Minimize GST wastage: The government should address the GST disparity between pharmaceutical inputs and final products, where inputs like KSMs and APIs are taxed at 18%, compared to 5% to 12% for final products, resulting in unutilized tax credits. Harmonizing GST rates by reducing input taxes and simplifying the IT Credit refund process within defined timelines (to avoid accumulation of tax credits) would ease this burden.
5. Improve port infrastructure and reduce logistics costs: India handles only 2% of global container traffic and lacks strong transshipment capabilities (75% of India’s transshipment cargo is handled at foreign ports). To enhance its role in the global pharmaceutical value chain, India must strengthen its port infrastructure by developing transshipment hubs, increasing draft depth, improving cold chain facilities, and ensuring end-to-end traceability, especially for temperature-sensitive products like vaccines and biosimilars. Additionally, to drive cost benefits for exporters, India needs to improve transportation of goods within the country. Domestic logistics costs in India total 13% to 14% of GDP, vs. 8% to 9% in developed economies, such as the US. The government should focus on reducing these costs by improving road infrastructure, opting for alternative fuels, and promoting transportation via inland waterways, where logistics costs are 60% lower than roadways.
Medium-term recommendations
1. Provide policy support for pharmaceuticals through a dedicated chapter in the FTP and infrastructure status: Establishing a specialized chapter for pharmaceuticals could help tailor trade policies and provide essential support to encourage exports. For example, e-commerce was introduced in India’s Foreign Trade Policy (FTP) in 2023 with the aim of promoting exports. Similarly, granting the pharmaceutical sector infrastructure status could allow private companies to borrow at lower rates with higher limits and to access foreign capital, thus driving export competitiveness.
2. Promote “Brand India”: The government should double down on its efforts to promote the Indian pharmaceutical industry’s brand through active participation in international events and cross-border roadshows. For instance, in October 2024, Pharmexcil organized the India Pavilion at the Convention for Pharmaceutical Ingredients (CPHI) in Milan. Further, iPHEX 2024, organized by Pharmexcil in India, hosted more than 500 delegates from over 100 countries with the objective of positioning “India as the Pharmacy of the World.” By having a strong brand presence at key upcoming exhibitions, India can build strategic global partnerships and engage with governments to advocate for lower regulatory and trade barriers. Further, to enhance access to key global markets, India should establish dedicated pharmaceutical export cells, including representatives from the Department of Pharmaceuticals, within relevant Indian embassies and high commissions abroad.
“India could consider positioning pharmaceutical experts through DoP at appropriate levels within embassies/high commissions abroad for key markets such as USA and UK. This would enhance local market insights, enable regulatory liasoning, and streamline market entry for Indian pharmaceuticals.”
3. Cultivate a robust innovation ecosystem: The Indian government should encourage collaboration among industry, top academic institutions, biotechnology firms, and biotech incubators to drive cutting-edge research. A successful model to consider is the New England innovation hub in the US, which integrates top universities like MIT and Harvard with major pharmaceutical players such as Moderna and Pfizer, fostering dynamic industry-academia collaborations. Additionally, India should accelerate talent development by providing skilling subsidies, nurturing basic sciences talent by funding universities, establishing world-class institutes, easing regulations to attract global universities to open satellite campuses, and encouraging foreign-educated researchers and scientists to return, spurring innovation.
“India must foster an innovation ecosystem anchored on industry, government, and academia, and supported by VC funding. Coupled with entrepreneurial spirit, this will accelerate innovation.”
4. Strictly enforce revised Schedule M: The government should enforce the revised Schedule M, which was introduced in December 2023 to ensure compliance with WHO GMPs. These revisions outline detailed guidelines for maintaining Pharmaceutical Quality Systems (PQS), Quality Risk Management (QRM), and validation processes. To support pharmaceutical companies, the government should provide adequate time and resources for implementation while maintaining strict oversight to guarantee adherence. Further, India—as the leader of Global South—can champion the WHO’s global regulatory authority on equal footing with US-FDA, thus benefiting from stringent Schedule M compliance.
“Airline industry has implemented stringent quality standards whereby the flight is grounded even if a single safety check fails. The same rigor is required in pharmaceuticals, given its impact on countless lives.”
Long-term recommendations
1. Adopt global guidelines to secure membership in organizations like ICH and PIC/S: India is currently an observer in the ICH with no decision-making rights. By adopting ICH guidelines, which are stricter than the Indian Pharmacopoeia Commission (IPC) standards, India can pave the way for future membership in ICH and PIC/S, which will enable the adoption of global best practices, streamline regulatory approvals, and boost the credibility of Indian pharmaceutical exports. This will especially help in the ASEAN region, where smaller Indian companies are failing to compete with local players who meet PIC/S standards
“The revised Schedule M positions India well to meet WHO GMP standards and potentially join PIC/S within 10 to 15 years, opening up the world to Indian pharma exports.”
These recommendations, while not exhaustive, will help position India as a pharmaceutical export hub. Currently, exports across sectors are facing headwinds, with concerns related to high interest rates, soaring freight charges, and the unavailability of shipping lines. Approximately 40% of respondents in a survey by the Federation of Indian Export Organizations expected a decline in exports, citing these concerns. However, the government has already taken some steps, namely, reducing port charges, purchasing additional container vessels, and expediting customs clearance to ease the burden.
“Concerns highlighted by Indian exporters—namely, interest rates and freight rates—are short term in nature and are unlikely to continue in the long-term. GoI has been proactive in terms of taking steps such as additional container vessels to alleviate concerns around unavailability of shipping lines.”
Short-term concerns aside, there are many encouraging signs, with the private sector and the government making strides toward realizing India’s long-term pharmaceutical growth potential. The mantra “QuRATE” aptly sums up the five overarching pillars that are critical to ensuring success for Indian pharma exports by 2047 (see Figure 15).

Taking a future-back view, if the value-driven transition needs to happen with India’s pharma exports reaching about $350 billion by 2047, some key swing factors across segments must converge in addition to “QuRATE” (see Figures 16a and 16b).
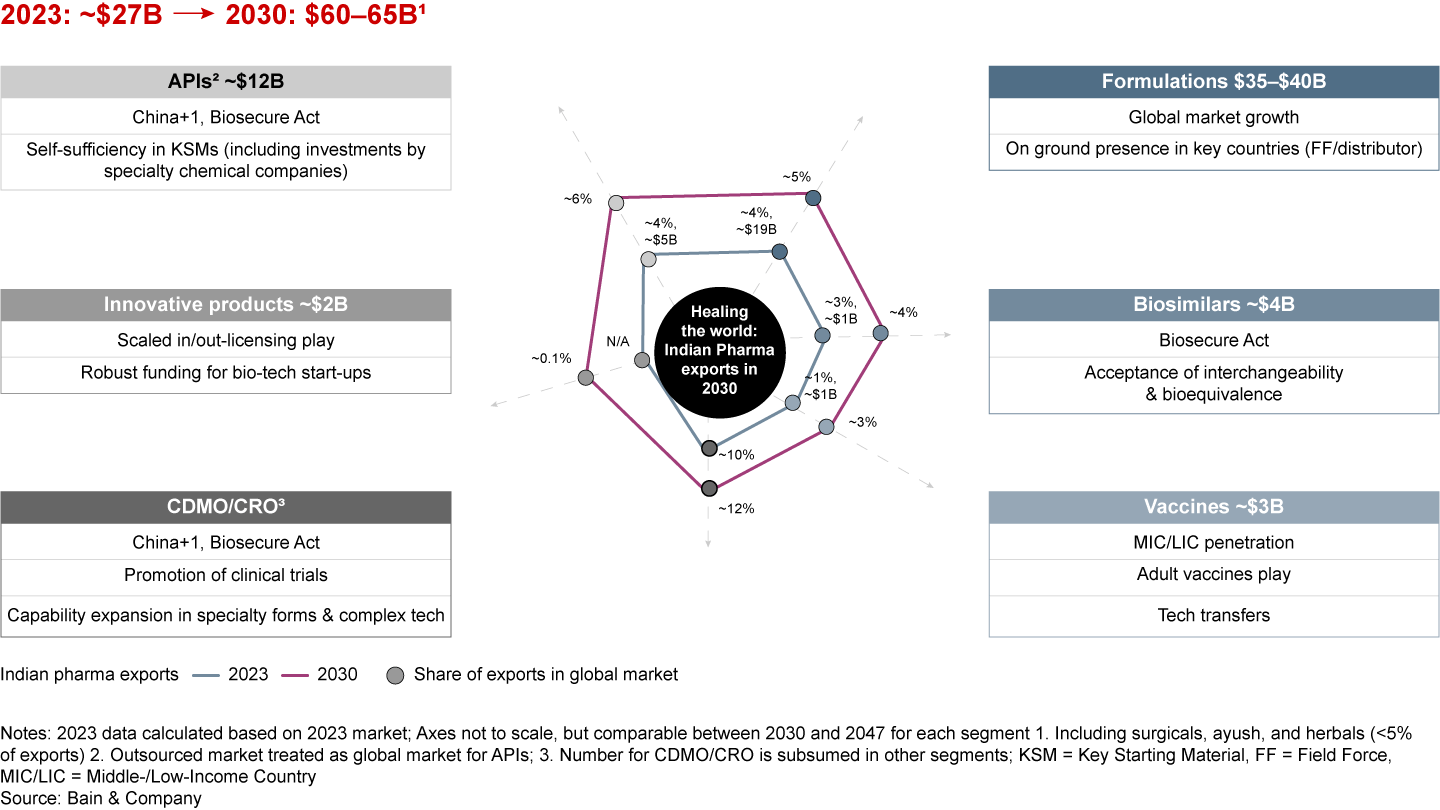

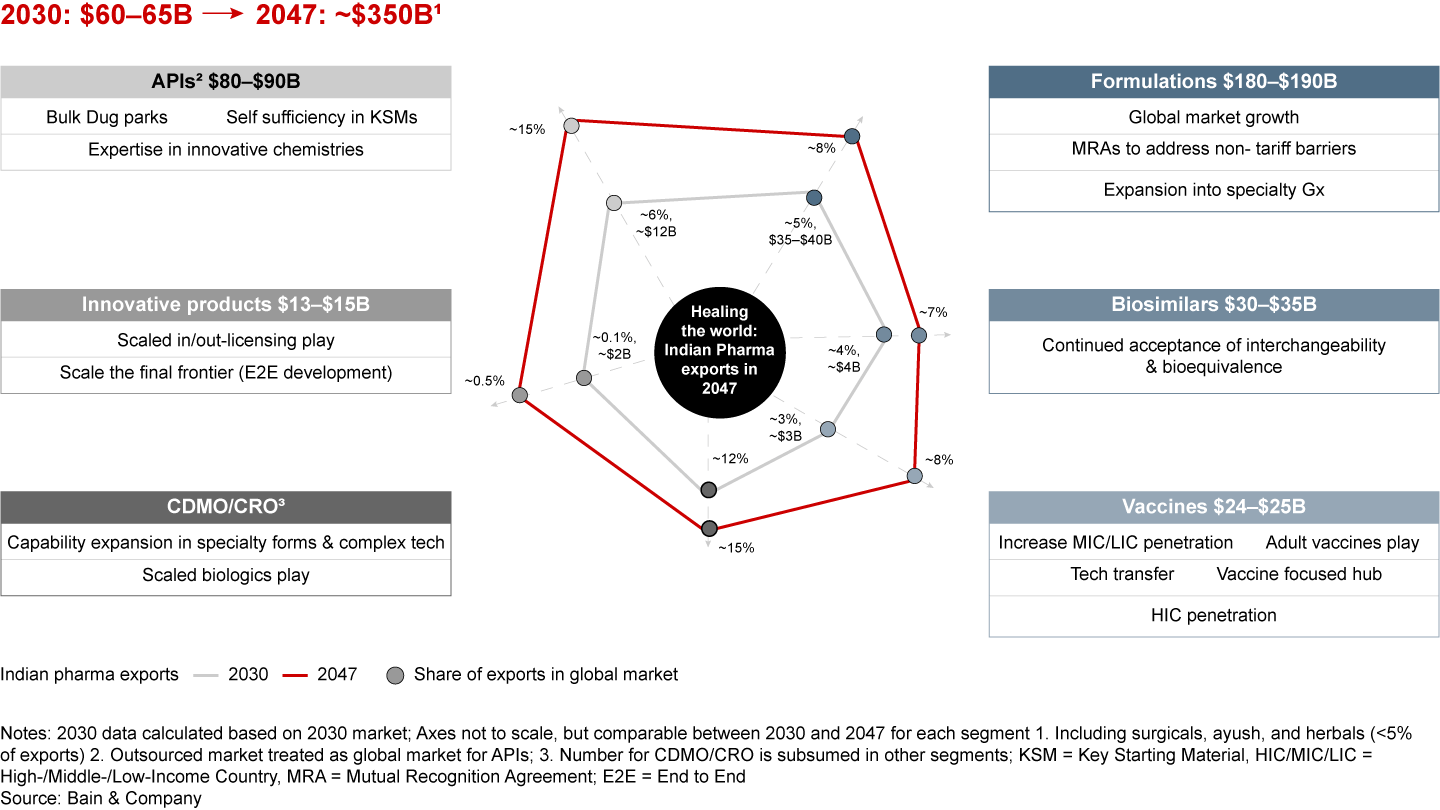

For instance, a successful expansion into specialty generics, coupled with MRAs that address non-tariff barriers, can unlock the next frontier of formulations growth. Doubling down on bulk drug parks, achieving self-sufficiency in KSMs, and advancing expertise in innovative chemistries can drive API growth. In innovative products, fostering entrepreneurial innovation and cultivating a robust talent pool could propel end-to-end product development and unshackle growth.
India’s journey from a fledgling pharma exporter to achieving 10-fold growth over two decades is a testament to its potential. With this strong trajectory, there is every reason to believe that India can reach even bigger goals. Realizing this vision will require all stakeholders across the private sector and the government to come together. By adopting a focused strategy, making targeted investments, and embracing the “QuRATE” mantra, India is poised to establish itself as the healthcare custodian of the world by 2047 and advance the vision of a Viksit Bharat (“Developed India”).

About IDMA
The Indian Drug Manufacturers’ Association (IDMA), established in 1961, has been instrumental in driving the Indian pharmaceutical industry’s growth, ensuring near self-sufficiency in affordable, quality medicines for India and the world. With approximately 1,000 members and eight State Boards across Tamil Nadu, Kerala and Puducherry, Gujarat, West Bengal, Haryana, Himachal Pradesh and Uttarakhand, Madhya Pradesh, Telangana and Karnataka, IDMA represents small, medium, and large-scale manufacturers nationwide. IDMA collaborates with regulators and government authorities on policy and regulatory issues, contributing to initiatives like Revised Schedule M (successfully achieved a one-year extension for MSMEs), drug and medical device pricing reforms, and the NMC Regulation, ensuring industry growth with a patient-centric focus.

About IPA
Indian Pharmaceutical Alliance (IPA) represents 23 leading Indian pharmaceutical companies committed to patient care in India and across the world. Collectively, IPA member companies account for over 85% of the private sector investment in pharmaceutical research and development. They contribute more than 80% of the exports of drugs and pharmaceuticals and service over 64% of the domestic market. The primary focus areas of IPA are innovation, quality, and global reach.

About Pharmexcil
The Pharmaceuticals Export Promotion Council of India (Pharmexcil) was established in 2004 by the Ministry of Commerce and Industry, Government of India, under the Foreign Trade Policy. Its primary goal is to promote and facilitate the export of Indian pharmaceutical products globally. Supporting over 4,200 members involved in formulations, APIs, biologics, and pharma services, Pharmexcil provides a platform for collaboration, market research, export promotion, and compliance with international regulations.