Brief

Auf einen Blick
- Global pharma supply chains have become increasingly vulnerable to disruption.
- Close to 40% of manufacturing sites for active pharmaceutical ingredients are located in India or China.
- The Asia-Pacific region accounts for 45% of pharmaceutical production but represents only 18% of demand value.
As Covid-19 squeezed off critical supplies of drug components from Asia, pharma companies faced a moment of truth. Their dependency on far-flung supply chains carried a perilous risk. It wasn’t the first warning. Tsunamis wrecked pharma manufacturing facilities in Asia in 2011 and 2012, and in 2017, Hurricane Maria knocked out Puerto Rico’s electricity supply, disabling many of the nearly 50 pharmaceutical plants on the island for months. But the pandemic finally has convinced leaders to build more resilience into their supply chains.
It’s a pivotal switch. For more than a decade, pharma companies have sought to lower costs by relocating a significant share of their manufacturing capacities, especially in the small molecules business, which includes active pharmaceutical ingredient (API) and drug production and packaging, to China and India. As a result, production volumes in these countries grew much faster than demand (see Figure 1). In 2019, close to 40% of registered manufacturing sites for APIs were located in India or China, according to US Food and Drug Administration (FDA) data.
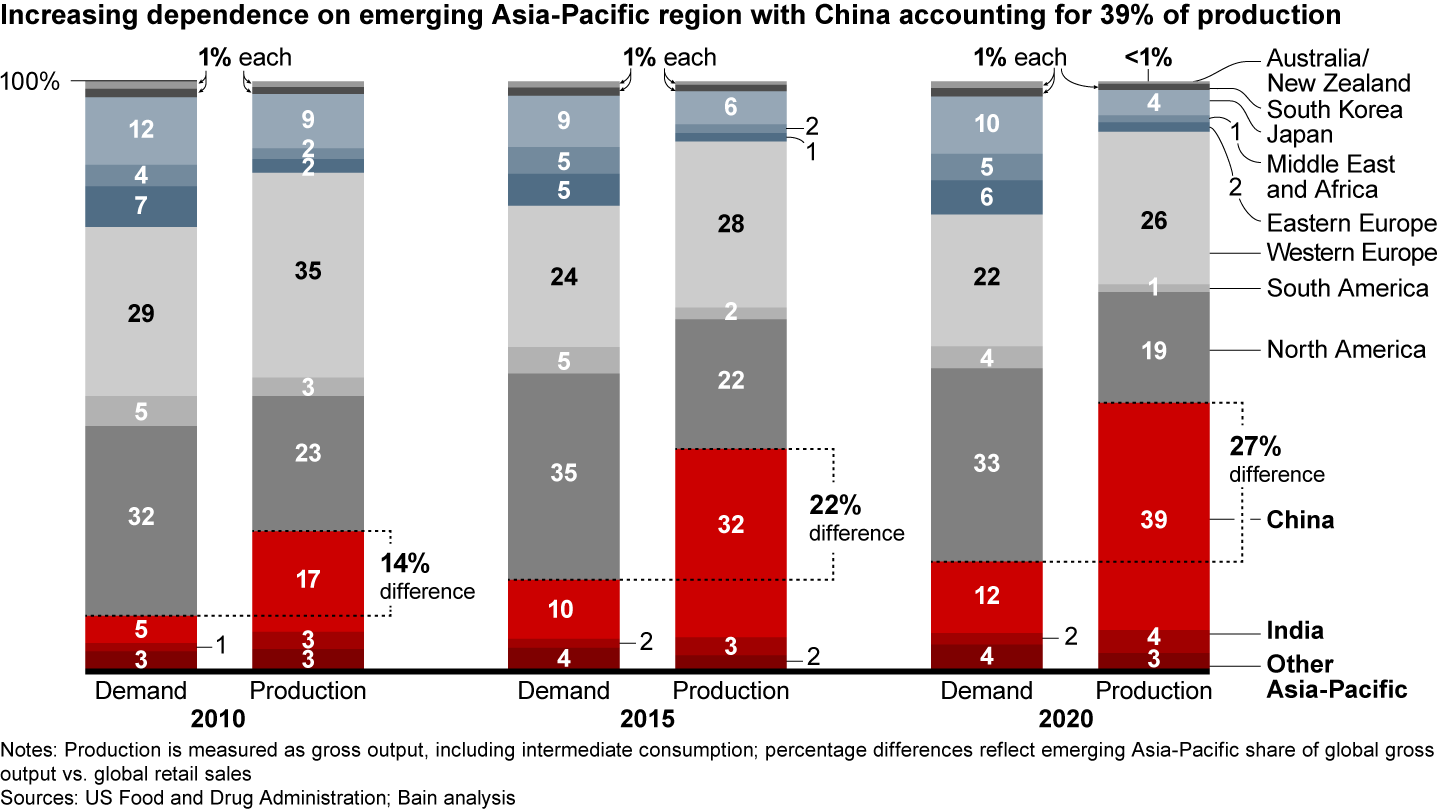

The transfer of pharma sourcing and manufacturing to Asia has directly affected supply chain reliability. FDA data shows an increase in drug shortages resulting from several factors, including quality issues and disruptive events, such as the 2017 fire at a Chinese API producer that led to a global shortage of piperacillin/tazobactam (see Figure 2). The winner-take-all generic pharmaceutical bidding process in Germany also has produced drug shortages by reducing the number of producers or bidders over time. In some cases, for example, the winners were sales offices dependent on foreign third-party manufacturers incapable of delivering high drug volumes.


Pandemic fallout
Covid-19 severely disrupted pharma supply chains, issuing a loud warning to the industry. While demand for critical care medications (anesthetics) and antidepressants surged, manufacturing site closures and impaired transportation routes immobilized supply chains. Pharmaceutical producers were unable to react fast enough to secure the supply they needed. Of the 118 FDA-reported drug shortages in August 2020, 55 were related to an increase in demand, 16 to API shortages, and 8 to manufacturing or shipping delays. The majority of drugs in short supply are small molecule generics. Only two of the FDA-listed shortages are biologicals (belatacept, asparaginase); two others are peptide hormones (sincalide, parathyroid hormone).
Government actions to safeguard local drug supplies have further strained pharma supply chains. For example, in March, India‘s Ministry of Commerce and Industry banned the export of 26 drugs—including paracetamol; antibiotics such as erythromycin, clindamycin, and metronidazole; and the antiviral acyclovir.
Drug shortages and over-reliance on API imports are not new developments, but the pandemic has underscored the growing risk and triggered public concern. Leading pharma companies are taking key steps to increase supply chain resilience, to ensure manufacturing continuity and patient safety, and to protect the business in times of uncertainty.
Building resilient pharma supply chains
As every pharma executive knows, moving production closer to home markets would be costly and would take years to accomplish. A more pragmatic two-step approach can increase the resilience of existing supply chains.
First, companies need a more complete understanding of the risks that span the entire supply chain (see Figure 3). To increase transparency, leadership teams map production sites, distribution center and material flows (including APIs, excipients and packaging) against potential hazards. They take into account geopolitical developments (such as trade wars, natural disasters, epidemics and strikes) that could affect supply and demand and lead to drug shortages. Companies can evaluate their risk exposure based on the level of impact and likelihood at both the overall company level and individual site level.
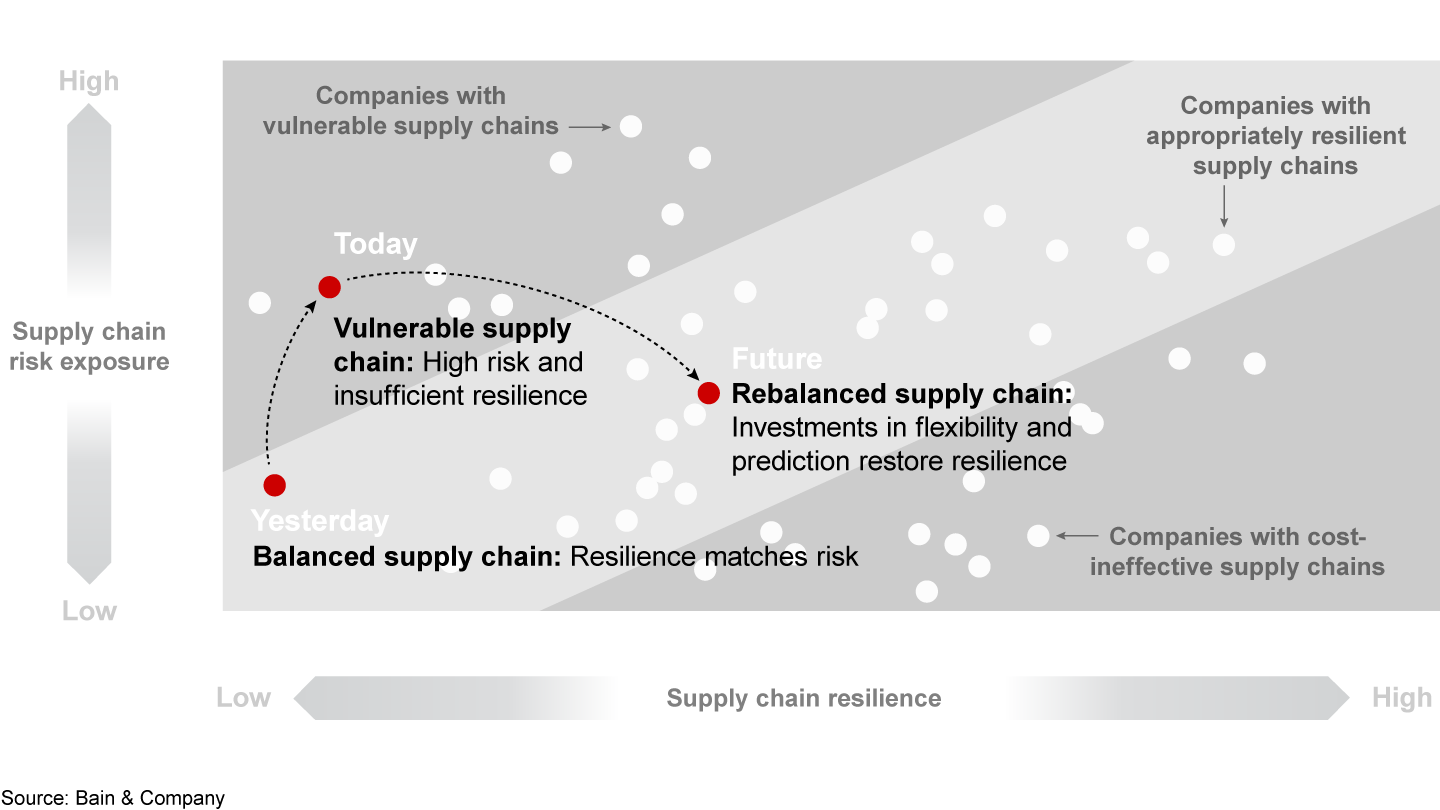

Second, based on the results of the risk assessments, leadership teams create new supply chain strategies by reducing the risk exposure or by increasing resilience capabilities—or by doing both. Effective strategies should include four vital components.
Redundancy: Building capacity buffers into manufacturing facilities enables companies to raise or lower production volume quickly. These buffers include safety stocks of critical drug components, flexible production lines with extra capacity, and shift models designed for rapid response, such as night shifts when needed, including a mix of employees and temporary staff.
Leaders create an agile production ecosystem through flexible contracts with suppliers and manufacturers so that they can shift production volume and location as needed within a broad array of options. To ensure a rapid response to market shortages, they develop multiple backup sources for key materials and multiple quality certification options.
Adaptability: A modular approach to manufacturing enables companies to shift production when needed to other sites across the globe. Increasing production line digitization and automation also bolster adaptability. That, in turn, supports operators when they need to make rapid changes to cope with disruption.
A third facet of adaptability is developing clear response plans to different scenarios. Pharma companies can react quickly to disruptions when they have already ranked SKUs by priority and set up fast, pragmatic decision-making processes to change investment plans quickly if necessary.
Prediction: Control tower technology and artificial intelligence solutions give leadership teams vastly improved visibility across the entire network; they also help predict demand fluctuations and risks. Leaders use those tools to provide better visibility to customers as well as suppliers, sharing real-time product demand and stock data. To identify potential weak links in the supply chain and move quickly from insight to action, these companies continuously analyze public data and simulate scenarios.
Empowerment: To get the most from resilient supply chains, successful companies bolster problem-solving capabilities throughout their organization and at manufacturing sites. Importantly, they empower local organizations to make decisions that can protect business continuity in the midst of a crisis.
Covid-19 has prompted a pivotal shift in pharma supply chain strategies. Over the coming decade, resilient networks will be key to navigating an increasingly turbulent market. Pharma companies that integrate flexibility and redundancy into the entire value chain and that improve visibility will be best positioned to predict chain disruptions efficiently and respond to them rapidly.

Coronavirus
The global Covid-19 pandemic has extracted a terrible human toll and spurred sweeping changes in the world economy. Across industries, executives have begun reassessing their strategies and repositioning their companies to thrive now and in the world beyond coronavirus.